Example Of The First Law Of Thermodynamics
Muz Play
Mar 29, 2025 · 7 min read
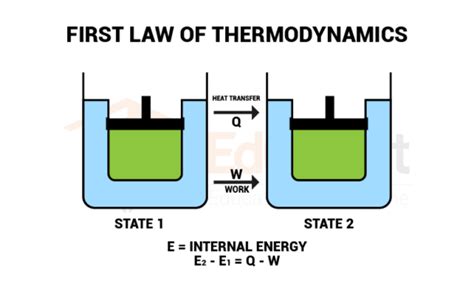
Table of Contents
Examples of the First Law of Thermodynamics: Energy Conservation in Action
The First Law of Thermodynamics, also known as the law of conservation of energy, is a fundamental principle in physics stating that energy cannot be created or destroyed, only transformed from one form to another. This seemingly simple statement underpins countless processes in the universe, from the smallest subatomic interactions to the largest cosmological events. Understanding its implications requires examining real-world examples that illustrate how energy changes form but remains constant in its total quantity. This article delves into various examples, categorizing them for clarity and providing detailed explanations.
Examples in Everyday Life
Many everyday occurrences demonstrate the First Law of Thermodynamics. These familiar examples make the abstract concept tangible and accessible.
1. Burning a Candle
A classic example is the burning of a candle. The chemical energy stored within the wax molecules is released as heat and light energy.
- Initial State: The candle wax contains potential chemical energy.
- Process: Combustion (burning) occurs, converting chemical energy into heat (thermal energy) and light (radiant energy). Some energy is also lost as sound energy (the crackling sound).
- Final State: The wax is consumed, releasing heat and light. The total energy remains constant; it has simply changed forms. The slight decrease in total energy is due to energy losses to the surroundings, such as heat radiating into the air.
This demonstrates that energy is neither created nor destroyed; the chemical energy in the wax is transformed into other forms of energy.
2. Boiling Water
Heating water on a stove provides another clear example.
- Initial State: The water possesses a certain amount of thermal energy at room temperature.
- Process: The stove burner transfers heat energy (thermal energy) to the water.
- Final State: The water's temperature increases, and eventually, it boils, converting some of the thermal energy into kinetic energy (movement of water molecules) and latent heat (energy required for phase change from liquid to gas). Some energy is lost to the surroundings as heat.
The total energy remains constant; the heat from the stove is transferred to the water, changing its thermal energy and state.
3. A Swinging Pendulum
A simple pendulum illustrates the interplay between potential and kinetic energy.
- Initial State: At its highest point, the pendulum bob possesses maximum potential energy (energy due to its position). It has minimal kinetic energy (energy of motion).
- Process: As the pendulum swings down, potential energy is converted into kinetic energy.
- Final State: At the lowest point, the pendulum bob has maximum kinetic energy and minimum potential energy. The process reverses as it swings back up.
Ignoring friction and air resistance, the total mechanical energy (sum of potential and kinetic energy) remains constant throughout the pendulum's motion. In reality, some energy is lost to friction and air resistance, converting some of the mechanical energy into thermal energy.
4. Charging a Battery
Charging a rechargeable battery involves converting electrical energy into chemical energy.
- Initial State: The battery has a low level of stored chemical energy.
- Process: An external power source (e.g., a wall outlet) provides electrical energy, which is used to drive a chemical reaction within the battery. This reaction stores energy in the form of chemical bonds.
- Final State: The battery now has a higher level of stored chemical energy.
The electrical energy supplied is transformed into chemical energy within the battery.
5. Photosynthesis
Plants use sunlight to convert light energy into chemical energy during photosynthesis.
- Initial State: Sunlight provides radiant energy; carbon dioxide and water are the initial reactants.
- Process: Chlorophyll in plant cells absorbs light energy and drives a chemical reaction that converts carbon dioxide and water into glucose (a sugar) and oxygen.
- Final State: The glucose stores chemical energy; oxygen is released as a byproduct.
The light energy is transformed into chemical energy stored within the glucose molecules.
Examples in Larger Systems and Processes
The First Law applies not just to everyday experiences but also to larger-scale systems and processes.
6. Power Plants
Power plants, regardless of their energy source (nuclear, fossil fuels, hydroelectric, solar, geothermal, wind), all demonstrate the First Law.
- Initial State: The energy source possesses a large amount of stored energy (e.g., nuclear fuel, fossil fuels, potential energy of water).
- Process: The power plant converts this stored energy into thermal energy, which is then used to generate steam. The steam drives turbines, converting thermal energy into mechanical energy. This mechanical energy then drives generators, converting mechanical energy into electrical energy.
- Final State: Electrical energy is distributed to consumers. Energy is lost as heat throughout the entire process due to inefficiencies.
The total energy remains constant, although significant energy is lost as heat during various stages of conversion.
7. Internal Combustion Engine
The internal combustion engine in a car is another excellent example.
- Initial State: The fuel (gasoline or diesel) contains chemical energy.
- Process: Combustion converts the chemical energy into thermal energy, expanding gases that push pistons, creating mechanical energy. This mechanical energy is then transferred to the wheels via a transmission.
- Final State: The car moves, utilizing mechanical energy. Significant energy is lost as heat and sound.
The chemical energy in the fuel is ultimately converted into the kinetic energy of the moving vehicle, with substantial energy losses to the environment.
8. Nuclear Reactions
Nuclear reactions, both fission and fusion, directly demonstrate mass-energy equivalence as described by Einstein's famous equation, E=mc².
- Initial State: Nuclear fuel (e.g., uranium for fission) contains a vast amount of energy stored in the nuclei of its atoms.
- Process: Fission involves splitting heavy atomic nuclei, releasing a tremendous amount of energy as heat and radiation. Fusion involves combining light atomic nuclei, also releasing a large amount of energy.
- Final State: The products of the reaction have slightly less mass than the initial reactants; this mass difference is converted into energy according to E=mc².
This shows that mass can be converted into energy, and vice-versa, upholding the principle of energy conservation.
9. Weather Systems
Weather patterns are driven by energy transformations.
- Initial State: The sun provides radiant energy.
- Process: The sun's energy heats the Earth's surface unevenly, causing air to rise and fall, creating wind and atmospheric pressure differences. Evaporation of water requires energy, while condensation releases energy.
- Final State: The resulting movement of air masses, precipitation, and temperature changes are all manifestations of energy transformations driven by solar radiation.
Solar energy drives the complex energy conversions involved in atmospheric processes.
10. Human Metabolism
Human bodies are complex energy conversion systems.
- Initial State: Food contains chemical energy.
- Process: Digestion breaks down food, releasing chemical energy. This energy is used for various bodily functions: muscle movement (kinetic energy), maintaining body temperature (thermal energy), nerve impulses (electrical energy), and biosynthesis (chemical energy).
- Final State: The body utilizes the chemical energy from food for all its activities. Excess energy may be stored as fat (chemical energy).
The chemical energy in food is transformed into various forms of energy to support life processes.
Limitations and Considerations
While the First Law is fundamental, it doesn't tell the whole story. It doesn't address:
- The direction of energy flow: The Second Law of Thermodynamics addresses the tendency for systems to move towards greater disorder (entropy).
- The efficiency of energy conversion: Energy transformations are never 100% efficient; some energy is always lost as heat.
- The quality of energy: Different forms of energy have different qualities; for example, high-temperature heat is more useful than low-temperature heat.
Conclusion
The First Law of Thermodynamics, the principle of energy conservation, is a cornerstone of physics. Numerous examples from everyday life to large-scale processes demonstrate its fundamental nature. While simple in its statement, its implications are profound and far-reaching, affecting our understanding of the universe at every level. Understanding this law is crucial for comprehending how energy is transformed and utilized in countless systems, from the burning of a candle to the functioning of power plants and even the workings of our own bodies. By exploring these examples, we gain a deeper appreciation for the universal nature and importance of energy conservation.
Latest Posts
Latest Posts
-
What Elements Make Up A Carbohydrate
Mar 31, 2025
-
How Many Protons Are In A Sulfur Atom
Mar 31, 2025
-
How Many Bases In A Codon
Mar 31, 2025
-
Glucose And Galactose Differ At Which Carbon
Mar 31, 2025
-
Write The Formulas For The Following Compounds
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Example Of The First Law Of Thermodynamics . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
