What Elements Make Up A Carbohydrate
Muz Play
Mar 31, 2025 · 5 min read
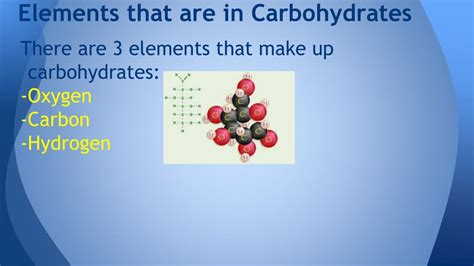
Table of Contents
What Elements Make Up a Carbohydrate? A Deep Dive into the Chemistry of Carbs
Carbohydrates. The word itself conjures images of pasta, bread, and sugary treats. But beyond their culinary appeal, carbohydrates are fundamental biomolecules crucial for life. Understanding their composition, structure, and function is key to appreciating their role in biology and human health. This article will delve into the elemental makeup of carbohydrates, exploring their various forms and implications.
The Building Blocks: Carbon, Hydrogen, and Oxygen
At its core, a carbohydrate is a compound composed of three elements: carbon (C), hydrogen (H), and oxygen (O). The ratio of hydrogen to oxygen atoms is typically 2:1, mirroring the ratio found in water (H₂O). This is why carbohydrates were historically called "hydrates of carbon," although this simplistic naming convention doesn't fully capture their complex structures and diverse functions.
The arrangement of these atoms is what differentiates various types of carbohydrates. This arrangement dictates their properties, such as solubility, digestibility, and the energy they provide.
The Empirical Formula: A Simplified Representation
The general empirical formula for carbohydrates is (CH₂O)ₙ, where 'n' represents the number of carbon atoms. This formula is a useful shorthand, but it’s important to note it doesn’t fully describe the structural variations that exist within the carbohydrate family. For example, while glucose (C₆H₁₂O₆) fits this formula, it differs structurally from fructose (also C₆H₁₂O₆) which is an isomer.
Classifying Carbohydrates: From Simple to Complex
Carbohydrates are broadly classified based on their structural complexity and the number of sugar units they contain. We can categorize them as:
1. Monosaccharides: The Simple Sugars
Monosaccharides are the simplest form of carbohydrates, also known as simple sugars. They are the fundamental building blocks of more complex carbohydrates. Key examples include:
- Glucose: Often called "blood sugar," glucose is a primary source of energy for cells throughout the body. It's found in fruits, honey, and other sweet foods.
- Fructose: Found abundantly in fruits and honey, fructose is sweeter than glucose. It's often used in processed foods as a sweetener.
- Galactose: Less commonly found on its own, galactose combines with glucose to form lactose, the sugar in milk.
These monosaccharides have a distinct ring structure, usually either a five-membered ring (furanose) or a six-membered ring (pyranose), depending on the arrangement of the atoms. The ring structure significantly impacts their reactivity and interactions with other molecules.
2. Disaccharides: Two Sugars United
Disaccharides are formed when two monosaccharides join together via a glycosidic bond. This bond is a covalent linkage between the carbon atoms of two monosaccharide molecules, often involving the removal of a water molecule (dehydration synthesis). Common disaccharides include:
- Sucrose (table sugar): A combination of glucose and fructose.
- Lactose (milk sugar): A combination of glucose and galactose.
- Maltose (malt sugar): A combination of two glucose molecules.
The properties of disaccharides, like sweetness and solubility, differ from their constituent monosaccharides. This is due to the change in the overall structure and the interactions of the combined monosaccharides.
3. Oligosaccharides: Short Chains of Sugars
Oligosaccharides consist of short chains of 3-10 monosaccharide units linked by glycosidic bonds. They are often found in beans, lentils, and other plant-based foods. While our bodies cannot directly digest many oligosaccharides, they act as prebiotics, feeding beneficial gut bacteria.
4. Polysaccharides: Long Chains of Sugar Units
Polysaccharides are complex carbohydrates composed of long chains of monosaccharides linked together by glycosidic bonds. They are the storage forms of energy and provide structural support in living organisms. Examples include:
- Starch: The primary storage form of glucose in plants. It consists of two main types: amylose (a linear chain) and amylopectin (a branched chain).
- Glycogen: The storage form of glucose in animals, primarily found in the liver and muscles. It has a highly branched structure, allowing for rapid glucose release when needed.
- Cellulose: The main structural component of plant cell walls. It's a linear polymer of glucose, but with a different glycosidic bond configuration than starch. Humans lack the enzymes to digest cellulose, making it dietary fiber.
- Chitin: A structural polysaccharide found in the exoskeletons of insects and crustaceans, as well as in the cell walls of fungi.
The different types of glycosidic bonds and the degree of branching in polysaccharides significantly influence their properties and digestibility.
Beyond the Basic Structure: Functional Groups and Modifications
While the basic formula (CH₂O)ₙ provides a foundation, the actual structure of carbohydrates is far richer. Functional groups like hydroxyl (-OH) and carbonyl (C=O) groups are key determinants of carbohydrate properties. The arrangement of these groups influences the formation of glycosidic bonds, the molecule's three-dimensional structure, and its interactions with other molecules.
Furthermore, carbohydrates can be modified by the addition of other chemical groups. For example, glycosylation, the addition of carbohydrate chains to proteins and lipids, profoundly impacts their function. These modifications are critical in cell signaling, protein folding, and immune system responses.
The Importance of Carbohydrates: Energy and Beyond
Carbohydrates are essential for various biological processes. Their primary role is energy provision. Glucose, derived from the digestion of carbohydrates, is metabolized through cellular respiration to generate ATP (adenosine triphosphate), the primary energy currency of cells.
Beyond energy production, carbohydrates perform crucial functions:
- Structural Support: Cellulose in plants and chitin in insects and fungi provide structural support.
- Cell Recognition and Signaling: Carbohydrates on cell surfaces participate in cell-cell recognition and communication, vital for immune function and other cellular processes.
- Glycosylation: The attachment of carbohydrates to proteins and lipids modifies their properties and functions, impacting numerous biological processes.
- Fiber: Dietary fiber, primarily composed of indigestible carbohydrates like cellulose, promotes healthy digestion and gut health.
Conclusion: A Diverse and Vital Class of Molecules
Carbohydrates, while often simplified as sugars, are a remarkably diverse and vital class of biomolecules. Their elemental composition—carbon, hydrogen, and oxygen—is the fundamental building block, but the arrangement of these atoms, along with functional group modifications, creates a vast array of structures with diverse roles in living organisms. Understanding the chemistry of carbohydrates is essential for appreciating their roles in energy production, structural support, and numerous other crucial biological processes. Their impact extends far beyond our plates, influencing cellular function and overall health in profound ways. From simple sugars to complex polysaccharides, the world of carbohydrates offers a rich landscape of chemical diversity and biological significance.
Latest Posts
Latest Posts
-
Area Between Two Polar Curves Formula
Apr 02, 2025
-
A Bronsted Lowry Base Is Defined As
Apr 02, 2025
-
Molecular And Empirical Formula Worksheet With Answers
Apr 02, 2025
-
How To Find The Maclaurin Series
Apr 02, 2025
-
How To Make Normal Probability Plot
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Elements Make Up A Carbohydrate . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
