Number Of Valence Electrons In Beryllium
Muz Play
Mar 18, 2025 · 6 min read
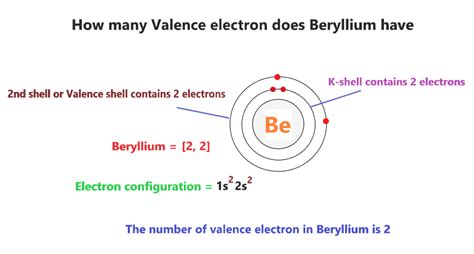
Table of Contents
The Enigmatic Valence Electrons of Beryllium: A Deep Dive
Beryllium, a fascinating element with the atomic number 4, often presents a unique challenge when discussing its valence electrons. While seemingly straightforward on the surface, a complete understanding requires delving into the intricacies of its electronic configuration and its behavior in chemical bonding. This article will provide a comprehensive exploration of the number of valence electrons in beryllium, exploring its implications in chemical reactivity and its overall significance in various scientific fields.
Understanding Valence Electrons: The Foundation
Before focusing specifically on beryllium, let's establish a firm understanding of what valence electrons are. Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. These electrons are the primary players in chemical bonding, determining how an atom will interact with other atoms to form molecules and compounds. Their number dictates the element's reactivity, oxidation states, and the types of bonds it can form (ionic, covalent, metallic).
The number of valence electrons is directly related to an element's position on the periodic table. Specifically, it's usually determined by the group (vertical column) to which the element belongs. However, transition metals and some other elements present exceptions to this general rule, making a deeper understanding of electronic configuration necessary.
Beryllium's Electronic Configuration: Unveiling the Mystery
Beryllium's atomic number is 4, meaning it possesses four protons and, in its neutral state, four electrons. The electronic configuration, which describes how these electrons are arranged in energy levels, is crucial for identifying the valence electrons. Beryllium's electronic configuration is 1s²2s².
Let's break this down:
- 1s²: This indicates that the first energy level (n=1) contains two electrons in the s orbital. The 's' orbital can hold a maximum of two electrons.
- 2s²: This indicates that the second energy level (n=2) also contains two electrons, both located in the s orbital of this level.
This is where the crux of the beryllium valence electron question lies. The outermost shell for beryllium is the second energy level (n=2). This shell contains two electrons. Therefore, beryllium possesses two valence electrons.
Why only two? The significance of shell filling.
It's important to note that while beryllium has four electrons, only the two in the outermost shell (2s²) are considered valence electrons. The inner shell electrons (1s²) are tightly bound to the nucleus and generally do not participate in chemical bonding. Their influence is primarily felt through shielding effects on the valence electrons.
Beryllium's Chemical Behavior: A Consequence of Two Valence Electrons
The presence of only two valence electrons significantly influences beryllium's chemical properties. This relatively small number of valence electrons leads to some distinctive characteristics:
1. Relatively High Ionization Energies:
Removing electrons from beryllium requires a significant amount of energy. Because the two valence electrons are relatively close to the nucleus, they experience a stronger electrostatic attraction and are, therefore, more difficult to remove compared to elements with more loosely held valence electrons.
2. Predominantly Covalent Bonding:
While beryllium can lose its two valence electrons to form Be²⁺ ions (forming ionic compounds in specific circumstances with highly electronegative elements such as oxygen and fluorine), it more commonly participates in covalent bonding. This means that it shares electrons with other atoms to achieve a stable electronic configuration, often forming linear or tetrahedral structures. The sharing of electrons helps to stabilize the atoms involved. Examples include BeCl2 and BeH2.
3. Limited Oxidation States:
The two valence electrons restrict beryllium to primarily having an oxidation state of +2. This means it tends to lose two electrons during chemical reactions. Higher oxidation states are extremely rare and observed under very specific conditions.
4. Amphoteric Nature in Oxides:
Beryllium oxide (BeO) exhibits amphoteric behavior, meaning it can react with both acids and bases. This amphoteric nature is influenced by the small size and high charge density of the Be²⁺ ion.
Beryllium's Applications: A Multifaceted Element
The unique properties of beryllium, stemming directly from its two valence electrons and resulting electronic structure, make it a valuable element in various applications:
- Aerospace Industry: Beryllium's high strength-to-weight ratio and rigidity make it ideal for lightweight aerospace components. Its low density allows for significant weight savings in aircraft and spacecraft.
- Nuclear Reactors: Beryllium's ability to moderate neutrons makes it useful as a neutron reflector in nuclear reactors. This property is crucial for controlling the nuclear chain reaction.
- X-ray Optics: Beryllium's low atomic number results in low X-ray absorption, making it suitable for constructing windows and mirrors for X-ray telescopes and other X-ray applications.
- Electronics: Beryllium alloys are used in high-precision instruments and electronic components due to their excellent dimensional stability and thermal conductivity.
Comparing Beryllium to its Neighbours: Understanding Periodic Trends
Examining beryllium's position within the periodic table in relation to its neighbors helps to further solidify the understanding of its valence electron configuration and its resulting properties.
- Lithium (Li): Located directly above beryllium, lithium has only one valence electron (1s¹2s¹). This single valence electron makes lithium significantly more reactive than beryllium. It readily loses this electron to form a +1 ion.
- Magnesium (Mg): Situated below beryllium in the same group (Group 2), magnesium, like beryllium, possesses two valence electrons (1s²2s²2p⁶3s²). However, due to the increased distance between the nucleus and the valence electrons, magnesium is generally more reactive than beryllium.
- Boron (B): To the right of beryllium, boron has three valence electrons (1s²2s²2p¹). This increased number of valence electrons gives boron different chemical properties, including a greater tendency to form covalent bonds with a variety of oxidation states.
Advanced Concepts: Beyond the Basics
While the simple understanding of two valence electrons provides a good foundation, a more in-depth analysis requires considering several sophisticated concepts:
- Hybridization: Beryllium's bonding often involves hybridization, where atomic orbitals combine to form new hybrid orbitals with different shapes and energies. This allows beryllium to form stable bonds with more than two atoms.
- Coordination Chemistry: Beryllium forms complexes with ligands (molecules or ions that bind to a central metal atom or ion). Understanding these complexes requires a deeper knowledge of coordination chemistry principles.
- Relativistic Effects: While less significant in beryllium compared to heavier elements, relativistic effects can slightly influence the electronic structure and properties of beryllium, especially when dealing with high-energy processes.
Conclusion: The Importance of Understanding Beryllium's Valence Electrons
The seemingly simple number of two valence electrons in beryllium has profound implications for its chemical behavior, reactivity, and its vast range of applications. A thorough understanding of beryllium's electronic configuration is essential for comprehending its unique properties and its important role across diverse scientific and technological fields. This article has explored the fundamental aspects of beryllium's valence electrons, highlighting their significance in shaping the element's character and its place in the broader world of chemistry and materials science. From the simplest chemical interactions to sophisticated technological applications, the influence of beryllium's two valence electrons remains undeniable.
Latest Posts
Latest Posts
-
How Much Energy To Be At Zero Kinetic Energy
Mar 18, 2025
-
Current As A Function Of Time
Mar 18, 2025
-
Ode To Billy Joe Lyrics Meaning
Mar 18, 2025
-
Where Does The Light Independent Reaction Take Place
Mar 18, 2025
-
S P D F Blocks On The Periodic Table
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Number Of Valence Electrons In Beryllium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
