Periodic Table Metals Metalloids And Nonmetals
Muz Play
Mar 23, 2025 · 6 min read
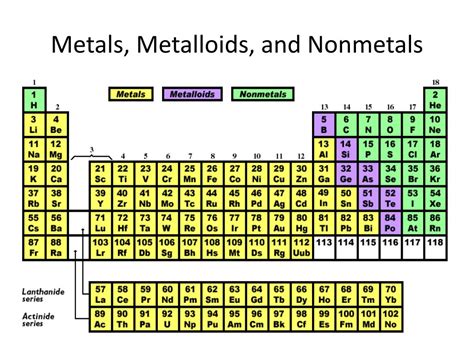
Table of Contents
The Periodic Table: A Deep Dive into Metals, Metalloids, and Nonmetals
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and recurring chemical properties. Understanding this organization is crucial to grasping the fundamental nature of matter. This article delves into the three major element categories – metals, metalloids, and nonmetals – exploring their characteristics, properties, and applications, enriched with SEO-friendly optimization techniques.
Understanding the Periodic Table's Organization
The periodic table's arrangement is not arbitrary. Elements are positioned according to their atomic number, which represents the number of protons in an atom's nucleus. This arrangement reflects a recurring pattern of properties, known as periodicity. Elements in the same column, or group, share similar chemical behavior due to having the same number of valence electrons – electrons in the outermost shell that participate in chemical bonding. Rows, or periods, represent elements with the same number of electron shells.
Key Features Driving Element Classification
Several key features dictate an element's classification as a metal, metalloid, or nonmetal:
- Electronegativity: This measures an atom's tendency to attract electrons in a chemical bond. Nonmetals generally exhibit high electronegativity, while metals display low electronegativity.
- Ionization Energy: This refers to the energy required to remove an electron from an atom. Metals typically have low ionization energies, while nonmetals have high ionization energies.
- Metallic Character: This describes the extent to which an element displays metallic properties, such as conductivity and malleability. Metals exhibit strong metallic character, while nonmetals lack it.
- Conductivity: Metals are excellent conductors of heat and electricity, a defining characteristic. Nonmetals are generally poor conductors.
Metals: The Dominant Group
Metals constitute the vast majority of elements on the periodic table. They are located on the left side and in the middle of the table. Their properties are largely defined by their tendency to lose electrons and form positive ions (cations).
Properties of Metals:
- Excellent Conductivity: Metals are renowned for their exceptional ability to conduct electricity and heat. This property is due to the freely moving electrons in their metallic bonding structure. This is why copper is widely used in electrical wiring and aluminum in cookware.
- Malleability and Ductility: Metals can be easily shaped (malleability) and drawn into wires (ductility). This is a direct consequence of the non-directional nature of metallic bonding. Gold, for instance, is highly malleable and can be hammered into extremely thin sheets.
- Luster: Most metals possess a characteristic metallic luster, a shiny appearance. This is due to the interaction of light with the free electrons in the metal's structure.
- High Density: Compared to nonmetals, metals generally exhibit higher densities. This is because of their closely packed atomic structures.
- High Melting and Boiling Points: Many metals possess high melting and boiling points, reflecting the strong metallic bonds holding their atoms together. However, there are exceptions, such as mercury, which is liquid at room temperature.
- Reactivity: The reactivity of metals varies greatly. Some metals, like alkali metals (Group 1), are extremely reactive with water and air, while others, like noble metals (e.g., gold, platinum), are very unreactive.
Common Examples and Applications of Metals:
- Iron (Fe): A crucial structural metal, used in construction, automobiles, and many other applications. Its alloys, such as steel, offer enhanced properties.
- Aluminum (Al): Lightweight and corrosion-resistant, aluminum is used extensively in aerospace, packaging, and building materials.
- Copper (Cu): An excellent conductor of electricity, copper is essential for electrical wiring and plumbing.
- Gold (Au) and Silver (Ag): Precious metals prized for their inertness, beauty, and conductivity. Used in jewelry, electronics, and investments.
- Titanium (Ti): A strong, lightweight, and corrosion-resistant metal, ideal for aerospace applications and biomedical implants.
Nonmetals: A Diverse Group with Varied Properties
Nonmetals are located on the upper right side of the periodic table. Unlike metals, they tend to gain electrons to form negative ions (anions). Their properties are diverse and vary significantly across the group.
Properties of Nonmetals:
- Poor Conductivity: Nonmetals are generally poor conductors of heat and electricity. Their electrons are tightly bound to their atoms, hindering electron movement.
- Brittle: Nonmetals are often brittle and lack the malleability and ductility of metals. They tend to shatter when subjected to stress.
- Low Density: Nonmetals generally have lower densities than metals.
- Low Melting and Boiling Points: Many nonmetals have relatively low melting and boiling points compared to metals.
- Varied Appearance: Nonmetals exhibit a wide range of appearances, from gases (oxygen, nitrogen) to solids (carbon, sulfur) with various colors and textures.
- High Electronegativity: Nonmetals typically have high electronegativity, strongly attracting electrons in chemical bonds. This leads to the formation of covalent bonds.
Common Examples and Applications of Nonmetals:
- Oxygen (O): Essential for respiration and combustion, oxygen is a vital component of the atmosphere.
- Nitrogen (N): A major constituent of the atmosphere, nitrogen is used in fertilizers and various industrial processes.
- Carbon (C): The basis of all organic compounds, carbon exists in various forms, including diamond, graphite, and fullerenes, each with unique properties.
- Chlorine (Cl): Used in water purification and as a component of many industrial chemicals.
- Sulfur (S): Used in the production of sulfuric acid, a crucial industrial chemical.
- Phosphorus (P): Essential for biological systems and used in fertilizers and detergents.
Metalloids: Bridging the Gap
Metalloids, also known as semimetals, occupy a fascinating middle ground between metals and nonmetals. They are located along the staircase-like line separating metals and nonmetals on the periodic table. Their properties are intermediate, exhibiting characteristics of both metals and nonmetals.
Properties of Metalloids:
- Semiconductor Properties: The defining characteristic of metalloids is their semiconducting behavior. Their electrical conductivity is intermediate, increasing with temperature, making them vital in electronics.
- Variable Appearance: Metalloids can exhibit various appearances, from metallic luster to non-metallic appearances.
- Brittle: Similar to nonmetals, metalloids are often brittle.
- Moderate Reactivity: Metalloids display moderate chemical reactivity, less reactive than most metals but more reactive than many nonmetals.
Common Examples and Applications of Metalloids:
- Silicon (Si): The most abundant metalloid, silicon is the foundation of modern electronics. It's used in semiconductors, solar cells, and computer chips.
- Germanium (Ge): Used in semiconductors, fiber optics, and various alloys.
- Arsenic (As): Used in semiconductors and various alloys. It's also used in pesticides (though this application is declining due to toxicity concerns).
- Antimony (Sb): Used in alloys, flame retardants, and various other applications.
- Tellurium (Te): Used in semiconductors and solar cells.
Conclusion: The Interplay of Properties and Applications
The periodic table's organization allows us to understand the relationships between elements and predict their behavior. The distinctions between metals, metalloids, and nonmetals are not always absolute; some elements exhibit properties that fall somewhere between categories. However, the general properties outlined above provide a valuable framework for understanding the diverse world of chemical elements and their numerous applications in various fields, from everyday materials to advanced technologies. Further exploration of the periodic table reveals even more intricate relationships and nuances in element properties and reactivity. Understanding these relationships is crucial for innovation and advancement in science and technology.
Latest Posts
Latest Posts
-
2 Sample Z Test For Proportions
Mar 25, 2025
-
Last Of Five Rhyming Greek Letters
Mar 25, 2025
-
Rusting Of Iron Is Chemical Or Physical Change
Mar 25, 2025
-
Differential Equation For Newtons Law Of Cooling
Mar 25, 2025
-
In Aerobic Cellular Respiration What Are The 3 Major Steps
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Periodic Table Metals Metalloids And Nonmetals . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
