Periodic Table Of Elements With Ionization Energy
Muz Play
Mar 28, 2025 · 6 min read
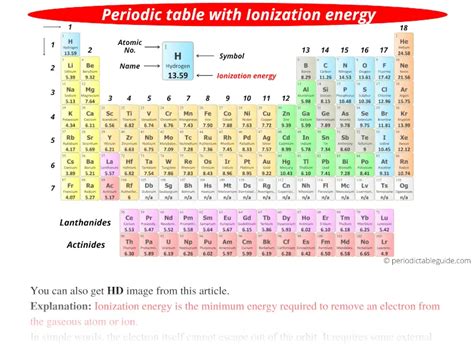
Table of Contents
The Periodic Table of Elements and Ionization Energy: A Deep Dive
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. One crucial property influencing an element's reactivity and behavior is its ionization energy. Understanding the relationship between the periodic table and ionization energy is fundamental to grasping the principles of chemical bonding, reactivity, and many other chemical phenomena. This comprehensive article delves into the intricacies of ionization energy, its trends across the periodic table, and its implications in various chemical contexts.
What is Ionization Energy?
Ionization energy (IE), also known as ionization potential, is the minimum amount of energy required to remove the most loosely bound electron from a neutral gaseous atom or molecule. This process results in the formation of a positively charged ion (cation). The first ionization energy (IE₁) refers to the energy needed to remove the first electron, the second ionization energy (IE₂) refers to the removal of the second electron from the singly charged ion, and so on. Each successive ionization energy is progressively larger because removing an electron from a positively charged ion requires overcoming the stronger electrostatic attraction between the remaining electrons and the nucleus.
In simpler terms: Imagine an atom as a miniature solar system, with electrons orbiting the nucleus. Ionization energy is the energy needed to "knock" an electron out of its orbit and completely remove it from the atom.
Trends in Ionization Energy Across the Periodic Table
Ionization energy isn't uniform across the periodic table; it exhibits distinct trends that reflect the underlying atomic structure. These trends are primarily governed by two factors:
-
Effective Nuclear Charge: This is the net positive charge experienced by the outermost electrons. A higher effective nuclear charge means a stronger attraction between the nucleus and the electrons, resulting in higher ionization energy. The effective nuclear charge increases across a period (left to right) due to increasing proton number, but remains relatively constant down a group (top to bottom) because of electron shielding.
-
Atomic Radius: The atomic radius represents the average distance between the nucleus and the outermost electrons. A smaller atomic radius leads to a stronger attraction between the nucleus and the electrons, thus resulting in higher ionization energy. Atomic radius generally decreases across a period and increases down a group.
Ionization Energy Trends Across Periods (Left to Right)
As you move across a period from left to right, the ionization energy generally increases. This is because:
- Increased Effective Nuclear Charge: The number of protons increases, leading to a stronger pull on the outermost electrons.
- Decreased Atomic Radius: The electrons are drawn closer to the nucleus, strengthening the attraction.
- Shielding Effect Remains Relatively Constant: The addition of electrons occurs within the same principal energy level, so the shielding effect by inner electrons does not significantly alter the trend.
This trend is reflected in the gradual increase in ionization energy values for elements within the same period.
Ionization Energy Trends Down Groups (Top to Bottom)
As you move down a group from top to bottom, the ionization energy generally decreases. This is due to:
- Increased Atomic Radius: The electrons are farther from the nucleus, weakening the attraction.
- Increased Shielding Effect: The addition of inner electron shells effectively shields the outermost electrons from the nuclear charge, reducing the effective nuclear charge.
This explains why elements lower down in a group exhibit lower ionization energies.
Exceptions to the General Trends
While the general trends are quite clear, there are some exceptions to the rules. These exceptions often arise due to:
-
Electron Configuration: Elements with half-filled or fully filled subshells (e.g., N, O, P, S) exhibit slightly higher ionization energies than expected due to extra stability associated with these configurations. The extra stability requires more energy to remove an electron.
-
Electron-Electron Repulsion: In some instances, the increased electron-electron repulsion can outweigh the increased effective nuclear charge, leading to a slight decrease in ionization energy. This is particularly evident in comparing elements with partially filled and fully filled orbitals.
Applications of Ionization Energy
Ionization energy is not just an abstract concept; it has significant practical applications in various fields:
-
Spectroscopy: The analysis of spectral lines, which are generated when electrons transition between energy levels, allows for the determination of ionization energies. This is crucial in identifying elements and understanding their atomic structure.
-
Chemical Bonding: Ionization energy provides insight into an element's tendency to form ions and participate in chemical bonding. Elements with low ionization energies readily lose electrons to form cations, while those with high ionization energies tend to gain electrons to form anions.
-
Material Science: Understanding ionization energy is crucial in designing materials with specific properties. For instance, the ionization energy of semiconductor materials determines their electrical conductivity and other electronic properties.
-
Atmospheric Chemistry: Ionization processes play a vital role in atmospheric chemistry, particularly in the formation of ions in the ionosphere. Ionization energy values help model these processes and understand their impact on the Earth's atmosphere.
Successive Ionization Energies: A Deeper Look
As mentioned earlier, successive ionization energies (IE₂, IE₃, etc.) progressively increase. The significant jump in ionization energy between successive ionizations can provide insights into the electron configuration of an element. For example, a large jump between IE₁ and IE₂ indicates that the first electron was removed from the valence shell, while the second electron was removed from a lower energy level, closer to the nucleus. This information aids in determining the number of valence electrons an element possesses.
Relationship Between Ionization Energy and Other Periodic Trends
Ionization energy is intrinsically linked to other periodic trends, including:
-
Electronegativity: Elements with high ionization energies tend to have high electronegativities, meaning they strongly attract electrons in a chemical bond.
-
Electron Affinity: While ionization energy focuses on removing electrons, electron affinity describes the energy change when an atom gains an electron. Elements with high ionization energies generally have negative (exothermic) electron affinities.
-
Metallic Character: Elements with low ionization energies are typically more metallic in character, readily losing electrons to form positive ions.
Conclusion: Ionization Energy as a Key to Understanding the Periodic Table
Ionization energy serves as a powerful tool for understanding the periodic table and the chemical behavior of elements. Its trends across periods and groups reflect the underlying atomic structure and the interplay between effective nuclear charge, atomic radius, and electron shielding. While general trends exist, exceptions highlight the complexities of electron configurations and inter-electronic repulsions. Ionization energy finds significant applications in various scientific disciplines, from spectroscopy and chemical bonding to material science and atmospheric chemistry. A thorough understanding of ionization energy is fundamental to grasping the principles of chemistry and its vast applications in the world around us. By studying the periodic trends of ionization energy, we gain crucial insights into the reactivity and behavior of elements and pave the way for further advancements in scientific research and technological development. Further exploration of ionization energy in conjunction with other periodic trends will undoubtedly continue to unveil deeper understanding of the fundamental properties that dictate chemical and physical behavior of elements.
Latest Posts
Latest Posts
-
When Do You Consider Log Diterminants Similar
Mar 31, 2025
-
Find The Rectangular Equation And Eliminate The Parameters
Mar 31, 2025
-
How To Calculate The Enthalpy Of Fusion
Mar 31, 2025
-
How Does Meiosis Generate Genetic Diversity
Mar 31, 2025
-
Truth Table With P Then Q
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Periodic Table Of Elements With Ionization Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
