Polymers Are Made Of Individual Subunits Called
Muz Play
Mar 25, 2025 · 6 min read
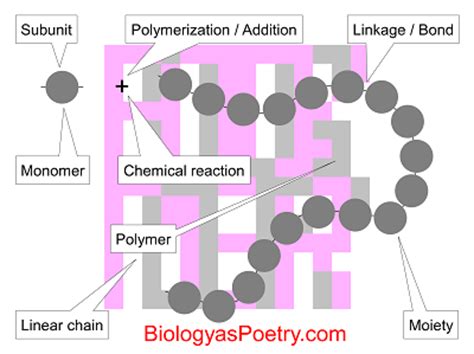
Table of Contents
Polymers Are Made of Individual Subunits Called Monomers: A Deep Dive into Polymer Chemistry
Polymers are everywhere. From the clothes on your back to the phone in your hand, these giant molecules play a crucial role in modern life. But what exactly are polymers? At their core, polymers are made of individual subunits called monomers. Understanding this fundamental relationship is key to grasping the incredible diversity and functionality of polymeric materials. This article will delve deep into the world of polymers, exploring the types of monomers, the processes of polymerization, and the resulting properties of the polymers they create.
What are Monomers?
Monomers are the building blocks of polymers. Think of them as individual LEGO bricks. These are relatively small molecules, often containing carbon, hydrogen, oxygen, nitrogen, or other elements. The crucial characteristic of a monomer is its ability to chemically bond with other monomers to form long chains. This bonding process is called polymerization. The chemical structure of the monomer dictates many of the properties of the resulting polymer. For example, a monomer with a rigid structure will often lead to a rigid polymer, while a monomer with flexible linkages will typically produce a more flexible polymer.
Examples of Common Monomers:
-
Ethylene (Ethene): The simplest monomer, ethylene, is the building block of polyethylene (PE), a ubiquitous plastic used in countless applications. Its simple structure – CH₂=CH₂ – allows for easy chain formation.
-
Propylene (Propene): Similar to ethylene, propylene (CH₂=CHCH₃) is another alkene monomer used to produce polypropylene (PP), known for its strength and versatility.
-
Styrene: Styrene (C₈H₈) is a vinyl monomer used to produce polystyrene (PS), a common material in packaging and insulation. Its aromatic ring contributes to the rigidity of polystyrene.
-
Vinyl Chloride: Vinyl chloride (C₂H₃Cl) is the monomer for polyvinyl chloride (PVC), a widely used plastic known for its durability and resistance to chemicals. The presence of chlorine significantly affects the polymer's properties.
-
Amino Acids: In the realm of biopolymers, amino acids are the monomers that form proteins. The sequence and type of amino acids determine the protein's unique three-dimensional structure and function.
-
Glucose: Glucose (C₆H₁₂O₆) is the monomer of starch and cellulose, essential carbohydrates in plants. The linkage between glucose units impacts the properties of these polysaccharides.
Types of Polymerization Reactions:
The process of joining monomers to form polymers is called polymerization. Several different mechanisms drive this process:
1. Addition Polymerization:
Addition polymerization involves the direct addition of monomers to each other without the loss of any atoms. This type of polymerization is characteristic of monomers containing double or triple bonds (unsaturated monomers). The process initiates with a free radical, an ion, or a metal complex, which then adds to the double bond of a monomer, creating a reactive intermediate that can react with another monomer, and so on. This chain reaction continues until the chain is terminated. Examples include the production of polyethylene and polypropylene.
Key characteristics of addition polymerization:
- Unsaturated monomers: Monomers with double or triple bonds are required.
- No byproduct: No small molecules are released during the reaction.
- Chain reaction: The reaction proceeds through a chain reaction mechanism.
- High molecular weight polymers: This method typically leads to high molecular weight polymers.
2. Condensation Polymerization:
Unlike addition polymerization, condensation polymerization involves the joining of monomers with the elimination of a small molecule, such as water or methanol. This type of polymerization is typical of monomers with two reactive functional groups, such as carboxylic acids and amines. The reaction occurs through a stepwise process, with each step involving the formation of a new bond and the release of a small molecule. Examples include the synthesis of nylon and polyester.
Key characteristics of condensation polymerization:
- Bifunctional monomers: Monomers with two reactive functional groups are needed.
- Byproduct formation: Small molecules, like water, are released during the reaction.
- Stepwise reaction: The reaction proceeds through a stepwise mechanism.
- Control over molecular weight: The molecular weight of the polymer can be more easily controlled in condensation polymerization.
3. Ring-Opening Polymerization:
Ring-opening polymerization involves the opening of cyclic monomers to form linear polymers. This type of polymerization is often catalyzed by acids, bases, or metal complexes. The resulting polymers can have a variety of structures and properties depending on the monomer and the catalyst used. Examples include the production of poly(ethylene oxide) and polycaprolactone.
Key characteristics of ring-opening polymerization:
- Cyclic monomers: Cyclic monomers are the starting materials.
- Catalyst required: A catalyst is usually necessary to initiate the reaction.
- Versatile polymers: This method can produce polymers with various properties.
Properties of Polymers: The Influence of Monomers
The properties of a polymer are strongly influenced by the structure of its constituent monomers. Several factors play a significant role:
1. Monomer Structure:
The chemical structure of the monomer, including its functional groups and the presence of any side chains, significantly impacts the polymer's properties. For example, the presence of bulky side groups can hinder chain packing, leading to a more flexible and less crystalline polymer.
2. Molecular Weight:
Higher molecular weight polymers generally exhibit increased strength, higher melting points, and improved mechanical properties. The molecular weight distribution also affects the polymer's overall properties.
3. Degree of Polymerization:
The degree of polymerization (DP) refers to the average number of monomer units in a polymer chain. A higher DP indicates a longer chain and often correlates with improved mechanical properties.
4. Crystallinity:
The ability of polymer chains to pack together in an ordered arrangement influences crystallinity. Highly crystalline polymers are typically stronger, stiffer, and more resistant to solvents than amorphous polymers. The monomer structure plays a critical role in determining the crystallinity of the polymer.
5. Branching and Crosslinking:
Branching refers to the presence of side chains along the polymer backbone. Crosslinking involves the formation of chemical bonds between different polymer chains. Both branching and crosslinking affect the polymer's flexibility, strength, and thermal properties. Crosslinking, in particular, can lead to thermoset polymers that are insoluble and infusible.
Applications of Polymers: A World Shaped by Monomers
The versatility of polymers stems from the wide range of monomers available and the ability to tailor their properties through polymerization techniques. Polymers are found in nearly every aspect of modern life, with applications including:
-
Packaging: Polyethylene (PE), polypropylene (PP), and polystyrene (PS) are commonly used in food packaging, films, and containers.
-
Textiles: Polyesters, nylons, and acrylics are used in clothing, carpets, and other textiles.
-
Construction: PVC, polyethylene, and other polymers are used in pipes, insulation, and building materials.
-
Automotive: Polymers are used in car parts, bumpers, dashboards, and interior components.
-
Electronics: Polymers are used in electronic devices as insulators, coatings, and substrates.
-
Biomedical Applications: Biocompatible polymers are used in medical implants, drug delivery systems, and tissue engineering.
Conclusion: The Monomer-Polymer Connection
The fundamental relationship between monomers and polymers is central to understanding the vast array of materials that shape our world. The choice of monomer, the polymerization method, and subsequent processing steps all contribute to the final properties and applications of a polymer. The seemingly simple building blocks—monomers—hold the key to unlocking the complexity and versatility of polymeric materials, impacting various industries and aspects of daily life. Continued research and innovation in polymer chemistry promise even more exciting advancements in the future, leading to new materials with enhanced properties and expanded applications. Further investigation into specific monomer types and their resulting polymer properties can uncover a wealth of knowledge about this crucial area of materials science. Exploring the effects of varying polymerization techniques on the final product is another avenue ripe for deeper study. The world of polymers is vast and continuously evolving, driven by the endless possibilities offered by their fundamental building blocks – the monomers.
Latest Posts
Latest Posts
-
What Are Alpha Beta And Gamma
Mar 28, 2025
-
What Is The Function Of The Atrioventricular Valves
Mar 28, 2025
-
Where Does Replication Take Place In A Eukaryotic Cell
Mar 28, 2025
-
Direct Gene Activation Involves A Second Messenger System
Mar 28, 2025
-
What Is The Radius Of Hydrogen
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Polymers Are Made Of Individual Subunits Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
