Properties Of Ionic And Covalent Compounds Lab
Muz Play
Mar 27, 2025 · 6 min read
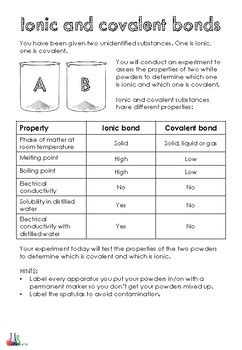
Table of Contents
Properties of Ionic and Covalent Compounds Lab: A Comprehensive Guide
This comprehensive guide delves into the fascinating world of ionic and covalent compounds, exploring their distinct properties through a detailed lab experiment analysis. We'll examine the fundamental differences between these two major classes of compounds and how these differences manifest in observable properties. Understanding these properties is crucial in chemistry, impacting fields ranging from materials science to medicine.
Understanding Ionic and Covalent Bonding
Before diving into the lab procedures and results, let's establish a solid foundation by reviewing the nature of ionic and covalent bonds.
Ionic Bonds: The Electrostatic Attraction
Ionic bonds arise from the electrostatic attraction between oppositely charged ions. This happens when one atom, typically a metal, readily loses electrons (becoming a positively charged cation), while another atom, often a nonmetal, readily gains those electrons (becoming a negatively charged anion). The strong coulombic forces holding these ions together form the ionic bond. Think of it like magnets: opposite charges attract!
Key Characteristics of Ionic Compounds:
- High melting and boiling points: The strong electrostatic forces require significant energy to overcome, resulting in high melting and boiling points.
- Crystalline structure: Ionic compounds typically form a highly ordered, three-dimensional crystal lattice structure. This structure maximizes the attractive forces between oppositely charged ions.
- Brittle nature: The rigid crystal lattice is susceptible to fracture. A slight shift in the crystal lattice can cause like charges to align, leading to repulsion and breakage.
- Conductivity: Ionic compounds conduct electricity when molten (liquid) or dissolved in water (aqueous solution). This is because the ions are free to move and carry charge. Solid ionic compounds, however, are poor conductors due to the fixed positions of the ions in the lattice.
- Solubility: The solubility of ionic compounds varies depending on the specific ions involved and the solvent used. Generally, polar solvents like water dissolve ionic compounds effectively due to the interaction between the polar solvent molecules and the charged ions.
Covalent Bonds: Sharing is Caring
Covalent bonds are formed when atoms share one or more pairs of electrons. This typically occurs between nonmetal atoms, where the electronegativity difference is relatively small. The shared electrons create a region of high electron density that holds the atoms together.
Key Characteristics of Covalent Compounds:
- Lower melting and boiling points: Compared to ionic compounds, covalent compounds generally have lower melting and boiling points. The intermolecular forces holding covalent molecules together are weaker than the electrostatic forces in ionic compounds.
- Variable physical states: Covalent compounds can exist as solids, liquids, or gases at room temperature depending on their molecular weight and intermolecular forces.
- Often non-conductive: Covalent compounds generally do not conduct electricity in either their solid or liquid states because they do not have free-moving charged particles. Some exceptions exist, especially in the case of acids that ionize in solution.
- Solubility: The solubility of covalent compounds depends heavily on the polarity of both the compound and the solvent. Polar covalent compounds tend to dissolve in polar solvents, while nonpolar covalent compounds dissolve in nonpolar solvents. This is described by the principle "like dissolves like."
The Lab Experiment: Investigating Properties
This lab experiment will focus on comparing and contrasting the properties of several ionic and covalent compounds. We'll use readily available materials and simple tests to demonstrate the key differences discussed earlier.
Materials Required:
- Several ionic compounds (e.g., NaCl, KCl, MgO)
- Several covalent compounds (e.g., sugar (sucrose), paraffin wax, iodine)
- Test tubes
- Bunsen burner or hot plate
- Test tube holder
- Beaker
- Distilled water
- Conductivity apparatus (e.g., a simple conductivity meter or a light bulb circuit)
- Spatula or scoop
Procedure:
1. Melting Point Determination:
Carefully heat small samples of each compound in separate test tubes using a Bunsen burner or hot plate. Observe and record the melting points (or decomposition points). Note: Some compounds might decompose before reaching their melting point.
2. Conductivity Test:
Test the conductivity of each compound in its solid state and then after dissolving a small amount in distilled water. Record your observations. Be cautious when handling hot materials.
3. Solubility Test:
Add a small amount of each compound to separate test tubes containing distilled water. Observe and record the solubility of each compound. Shake the tubes gently to aid dissolution.
4. Brittleness Test (for solid samples):
Carefully attempt to crush small samples of the solid ionic and covalent compounds. Observe and note any brittleness or flexibility.
Results and Discussion:
The results should clearly demonstrate the differences in properties between ionic and covalent compounds.
Expected Results:
- Melting Point: Ionic compounds will generally exhibit much higher melting points than covalent compounds.
- Conductivity: Ionic compounds will conduct electricity when dissolved in water or molten, but not in their solid state. Covalent compounds generally will not conduct electricity, whether dissolved or in solid form.
- Solubility: Solubility will vary depending on the polarity of the compound and the solvent. Ionic compounds often dissolve well in polar solvents like water. The solubility of covalent compounds depends on their polarity.
- Brittleness: Ionic compounds tend to be brittle, while many covalent compounds may show more flexibility.
Analyzing the Results:
Interpreting Melting Points: The high melting points of ionic compounds reflect the strong electrostatic attractions between ions in their crystal lattice. The lower melting points of covalent compounds result from the weaker intermolecular forces.
Understanding Conductivity: The conductivity of dissolved ionic compounds illustrates the presence of mobile ions carrying the electric charge. The non-conductivity of covalent compounds indicates the absence of free ions or electrons to conduct electricity.
Explaining Solubility: The solubility differences highlight the importance of "like dissolves like." Polar solvents effectively solvate ionic compounds and polar covalent compounds due to the strong interactions between the charged or polar species.
Interpreting Brittleness: The brittle nature of many ionic compounds is a direct result of the rigid, ordered structure of the crystal lattice. Disrupting this structure with even a small amount of force can cause the ions to repel and cause the crystal to fracture.
Extending the Lab: Advanced Investigations
This basic lab can be extended to include more sophisticated techniques and analyses. For example:
- Crystal Structure Visualization: Microscopic examination of the crystalline structures of ionic compounds can provide visual confirmation of the ordered lattice arrangement.
- pH Measurements: Determining the pH of aqueous solutions of ionic compounds can reveal whether the compound is acidic, basic, or neutral.
- Spectroscopic Analysis: Spectroscopic techniques such as infrared (IR) or nuclear magnetic resonance (NMR) spectroscopy can provide detailed information about the bonding and molecular structure of the compounds.
- Advanced Conductivity Measurements: Using more precise conductivity meters can provide quantitative data on the conductivity of solutions, allowing for comparisons and analysis of ionic mobility.
Conclusion:
This lab experiment offers a practical and insightful exploration into the contrasting properties of ionic and covalent compounds. By observing and analyzing the melting points, conductivity, solubility, and brittleness of various substances, students can gain a deeper understanding of the fundamental differences between ionic and covalent bonding and their influence on the macroscopic properties of matter. This knowledge forms a critical basis for further studies in chemistry and related disciplines. Remember to always practice safety procedures and handle chemicals responsibly during any laboratory work.
Latest Posts
Latest Posts
-
Calculate Ph At The Equivalence Point
Mar 30, 2025
-
What Happens When A Hydrate Is Heated
Mar 30, 2025
-
How Does Fermentation Allow Glycolysis To Continue
Mar 30, 2025
-
Which Subatomic Particle Carries A Positive Charge
Mar 30, 2025
-
Signs A Chemical Reaction Has Occurred
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Properties Of Ionic And Covalent Compounds Lab . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
