How Does Fermentation Allow Glycolysis To Continue
Muz Play
Mar 30, 2025 · 6 min read
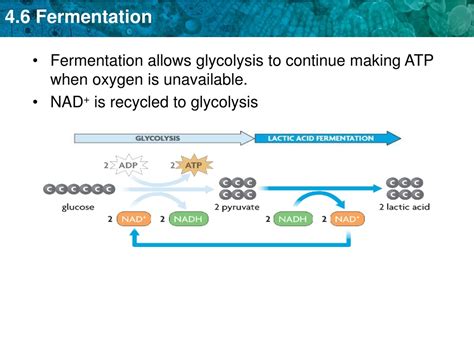
Table of Contents
How Does Fermentation Allow Glycolysis to Continue?
Glycolysis, the metabolic pathway that breaks down glucose into pyruvate, is a fundamental process for energy production in all living organisms. While aerobic respiration, utilizing oxygen as the final electron acceptor, is the most efficient way to extract energy from glucose, glycolysis can also proceed under anaerobic conditions—in the absence of oxygen—through a process called fermentation. This article delves deep into the intricate mechanism of how fermentation allows glycolysis to continue when oxygen is unavailable, exploring its different types, significance, and applications.
Understanding the Interplay Between Glycolysis and Fermentation
Glycolysis, a ten-step process occurring in the cytoplasm, yields a net gain of two ATP (adenosine triphosphate) molecules and two NADH (nicotinamide adenine dinucleotide) molecules per glucose molecule. NADH is a crucial electron carrier, playing a pivotal role in subsequent energy-generating processes. In aerobic respiration, NADH donates its electrons to the electron transport chain, ultimately leading to the production of a significantly larger amount of ATP through oxidative phosphorylation. However, this process requires oxygen as the final electron acceptor.
The critical link between glycolysis and fermentation lies in the regeneration of NAD+. NAD+ is the oxidized form of NADH, and it's essential for the continued operation of glycolysis. The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) enzyme, a key player in glycolysis, requires NAD+ to oxidize glyceraldehyde-3-phosphate. Without sufficient NAD+, glycolysis grinds to a halt because the reaction catalyzed by GAPDH becomes irreversible.
Fermentation solves this problem by providing an alternative pathway for NADH oxidation, regenerating NAD+ which can then be reused in glycolysis. This allows glycolysis to continue producing a small amount of ATP even in the absence of oxygen, ensuring the cell's survival under anaerobic conditions.
The Different Types of Fermentation
Fermentation encompasses various pathways, each yielding different end products. The two most common types are:
1. Lactic Acid Fermentation
Lactic acid fermentation is a relatively simple process where pyruvate, the end product of glycolysis, is directly reduced by NADH to form lactic acid. This reaction regenerates NAD+, enabling glycolysis to proceed.
The Biochemical Reaction:
Pyruvate + NADH + H⁺ → Lactate + NAD⁺
Lactic acid fermentation is prevalent in certain bacteria (like Lactobacillus) and also occurs in muscle cells during strenuous exercise when oxygen supply is insufficient. The build-up of lactic acid in muscles leads to muscle fatigue and soreness.
2. Alcoholic Fermentation
Alcoholic fermentation, predominantly carried out by yeasts (like Saccharomyces cerevisiae), involves a two-step process:
Step 1: Decarboxylation of Pyruvate:
Pyruvate is first decarboxylated by the enzyme pyruvate decarboxylase, releasing carbon dioxide (CO₂) and forming acetaldehyde.
The Biochemical Reaction:
Pyruvate → Acetaldehyde + CO₂
Step 2: Reduction of Acetaldehyde:
Acetaldehyde is then reduced by NADH to form ethanol, regenerating NAD+.
The Biochemical Reaction:
Acetaldehyde + NADH + H⁺ → Ethanol + NAD⁺
Alcoholic fermentation is responsible for the production of alcoholic beverages and also contributes to the rising of bread dough due to the production of CO₂.
Other Fermentation Pathways
Beyond lactic acid and alcoholic fermentation, various other types of fermentation exist, each characterized by unique end products and the specific enzymes involved. These include:
- Propionic acid fermentation: Produces propionic acid, acetic acid, and carbon dioxide. Common in Swiss cheese production.
- Butyric acid fermentation: Produces butyric acid, a volatile fatty acid with a characteristic rancid odor. Found in some anaerobic bacteria.
- Mixed acid fermentation: Produces a mixture of acids, including lactic acid, acetic acid, formic acid, and succinic acid. Typical of Escherichia coli.
Significance of Fermentation
Fermentation, despite its lower ATP yield compared to aerobic respiration, holds significant biological importance:
- ATP Production Under Anaerobic Conditions: It provides a crucial mechanism for ATP generation when oxygen is scarce, ensuring cellular survival in environments lacking oxygen.
- Food Preservation: Fermentation is extensively used in food preservation techniques, creating products like yogurt, cheese, sauerkraut, kimchi, and pickles. The acidic environment produced by fermentation inhibits the growth of spoilage microorganisms.
- Industrial Applications: Fermentation plays a vital role in various industrial processes, including the production of ethanol biofuels, pharmaceuticals (like antibiotics), and various enzymes.
- Metabolic Flexibility: Organisms capable of both fermentation and aerobic respiration exhibit metabolic flexibility, adapting their energy production pathways based on oxygen availability.
Fermentation and the Regulation of Glycolysis
The regulation of glycolysis is tightly controlled to maintain cellular energy homeostasis. While fermentation allows glycolysis to continue in the absence of oxygen, the overall rate of glycolysis is still regulated by various factors, including:
- Substrate Availability: The concentration of glucose and other glycolytic intermediates influences the rate of glycolysis.
- Enzyme Activity: The activity of key glycolytic enzymes, such as hexokinase, phosphofructokinase, and pyruvate kinase, is modulated by allosteric regulation and covalent modification.
- Energy Charge: The ratio of ATP to ADP and AMP acts as a sensor of the cell's energy status, affecting the activity of glycolytic enzymes. High ATP levels inhibit glycolysis, while low ATP levels stimulate it.
- Oxygen Availability: The presence or absence of oxygen directly impacts the fate of pyruvate, determining whether it proceeds to aerobic respiration or fermentation.
Fermentation: A Critical Evolutionary Adaptation
Fermentation likely emerged early in the evolution of life, providing a vital mechanism for energy production before the evolution of oxygenic photosynthesis and aerobic respiration. Its persistence in modern organisms highlights its crucial role in adapting to anaerobic environments and supporting a diverse range of metabolic processes.
Practical Applications and Future Research
The understanding of fermentation processes has led to numerous applications across various fields, including:
- Biofuel Production: Ethanol produced via alcoholic fermentation is a promising renewable biofuel source. Research focuses on improving the efficiency and sustainability of ethanol production.
- Food Science and Technology: Fermentation techniques are continuously refined to enhance the quality, safety, and nutritional value of fermented foods.
- Bioremediation: Microorganisms capable of fermentation are used in bioremediation efforts to clean up environmental pollutants.
- Pharmaceutical Industry: Fermentation is widely used in the production of antibiotics, vitamins, and other pharmaceuticals.
Ongoing research continues to explore the diversity of fermentation pathways, the genetic regulation of fermentation processes, and potential applications in various industries. Improving the efficiency and yield of fermentation processes remains a significant area of focus, with potential benefits in renewable energy production, food security, and environmental sustainability.
Conclusion
Fermentation is a crucial metabolic process that allows glycolysis to continue in the absence of oxygen. By providing an alternative pathway for NADH oxidation and regenerating NAD+, fermentation ensures the continued production of ATP, albeit at a lower yield compared to aerobic respiration. Its diverse applications in food production, biofuel generation, and various industrial processes highlight its significance in both biological and technological contexts. Further research into fermentation pathways and their regulation continues to unveil new possibilities and applications, contributing to advancements in various fields. The intricate interplay between glycolysis and fermentation is a testament to the remarkable adaptability and efficiency of cellular metabolism.
Latest Posts
Latest Posts
-
How Many Times Smaller Is An Electron Than A Proton
Apr 01, 2025
-
Which Homeostatic Process Moves Particles Against A Concentration Gradient
Apr 01, 2025
-
What Is The Intersection Of A Line And A Plane
Apr 01, 2025
-
Balance Sheet Of A Sole Proprietorship
Apr 01, 2025
-
What Magnification Is The Ocular Lens
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How Does Fermentation Allow Glycolysis To Continue . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
