Properties Of Systems In Chemical Equilibrium
Muz Play
Mar 25, 2025 · 6 min read
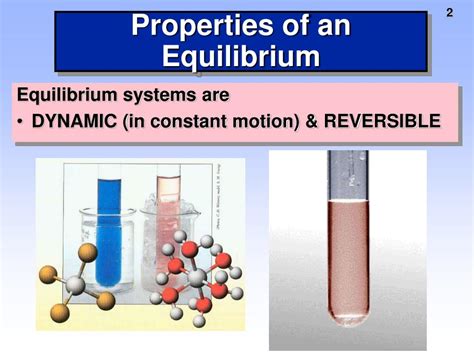
Table of Contents
Properties of Systems in Chemical Equilibrium
Chemical equilibrium is a dynamic state where the rates of the forward and reverse reactions are equal, resulting in no net change in the concentrations of reactants and products over time. Understanding the properties of systems at equilibrium is crucial in various fields, from industrial chemistry to environmental science. This article delves deep into the characteristics of systems in chemical equilibrium, exploring key concepts and their implications.
The Dynamic Nature of Equilibrium
It's crucial to emphasize that equilibrium is not static; it's a dynamic process. At equilibrium, both the forward and reverse reactions continue to occur at the same rate. This means that molecules of reactants are constantly transforming into products, and vice versa. However, the net change in concentration of reactants and products is zero. Think of it like a busy highway with equal traffic flowing in both directions – the overall number of cars remains constant, even though cars are constantly moving.
Microscopic Reversibility
This dynamic nature is directly related to the principle of microscopic reversibility. At equilibrium, each elementary step in a reaction mechanism proceeds at the same rate in both the forward and reverse directions. This ensures that the overall reaction rate remains balanced.
Key Characteristics of Systems at Equilibrium
Several characteristics define systems in chemical equilibrium:
1. Constant Macroscopic Properties:
At equilibrium, macroscopic properties like concentration, pressure (for gaseous systems), and temperature remain constant over time. This is because the rates of the forward and reverse reactions are equal, leading to no net change in these observable properties.
2. Dependence on Initial Conditions:
While equilibrium properties are constant over time, they do depend on the initial conditions of the system. Starting with different concentrations of reactants will lead to a different equilibrium composition, although the equilibrium constant (discussed below) will remain the same at a constant temperature.
3. Reversibility:
A system at equilibrium can be shifted in either direction by altering the conditions. This is known as Le Chatelier's principle, which states that a system at equilibrium will shift to relieve stress applied to it. This stress can be a change in concentration, pressure, temperature, or the addition of a catalyst.
4. Equilibrium Constant (K):
The equilibrium constant (K) is a crucial characteristic of a system at equilibrium. It's a ratio of the concentrations (or partial pressures for gases) of products to reactants, each raised to the power of its stoichiometric coefficient in the balanced chemical equation. For the general reaction:
aA + bB ⇌ cC + dD
The equilibrium constant expression is:
K = ([C]^c[D]^d) / ([A]^a[B]^b)
where [A], [B], [C], and [D] represent the equilibrium concentrations of the respective species. The value of K indicates the extent of the reaction at equilibrium:
- K >> 1: The equilibrium lies far to the right, favoring the products.
- K ≈ 1: The equilibrium lies roughly in the middle, with significant amounts of both reactants and products.
- K << 1: The equilibrium lies far to the left, favoring the reactants.
5. Temperature Dependence:
The equilibrium constant is highly sensitive to temperature changes. The effect of temperature on K is governed by the enthalpy change (ΔH) of the reaction. For exothermic reactions (ΔH < 0), increasing the temperature decreases K, shifting the equilibrium to the left. For endothermic reactions (ΔH > 0), increasing the temperature increases K, shifting the equilibrium to the right. This relationship is quantitatively described by the van't Hoff equation.
6. Independence of Catalyst:
A catalyst increases the rates of both the forward and reverse reactions equally, thus accelerating the attainment of equilibrium but not affecting the equilibrium position itself. The equilibrium constant remains unchanged in the presence of a catalyst.
Factors Affecting Equilibrium: Le Chatelier's Principle
Le Chatelier's principle provides a qualitative understanding of how changes in external conditions affect a system at equilibrium. The principle states that if a change of condition is applied to a system in equilibrium, the system will shift in a direction that relieves the stress. Let's examine how various changes influence the equilibrium:
1. Changes in Concentration:
Adding more reactant will shift the equilibrium to the right, favoring product formation. Conversely, adding more product will shift the equilibrium to the left, favoring reactant formation. Removing a reactant or product will have the opposite effect.
2. Changes in Pressure:
Changes in pressure primarily affect gaseous systems. Increasing the pressure favors the side of the reaction with fewer gas molecules, while decreasing the pressure favors the side with more gas molecules. If the number of gas molecules is the same on both sides, pressure changes will have no effect.
3. Changes in Temperature:
As discussed earlier, temperature changes affect the equilibrium constant itself. Increasing temperature favors the endothermic reaction (the reaction that absorbs heat), while decreasing temperature favors the exothermic reaction (the reaction that releases heat).
4. Addition of a Catalyst:
As mentioned earlier, a catalyst does not affect the equilibrium position. It only speeds up the rate at which equilibrium is reached.
Applications of Chemical Equilibrium
The principles of chemical equilibrium are essential in numerous applications across various scientific and technological fields:
1. Industrial Processes:
Many industrial processes, such as the Haber-Bosch process for ammonia synthesis and the production of sulfuric acid, rely heavily on controlling chemical equilibrium to maximize product yield and efficiency.
2. Environmental Chemistry:
Understanding equilibrium concepts is vital in assessing environmental issues like acid rain, the solubility of pollutants in water, and the distribution of chemicals in different environmental compartments.
3. Biochemistry:
Biological systems are constantly operating near equilibrium, and many biochemical reactions are governed by equilibrium principles. Enzyme kinetics, for instance, often involves analyzing reaction rates and equilibrium states.
4. Pharmaceutical Chemistry:
Drug design and development frequently involve considerations of chemical equilibrium to ensure that drugs reach their target sites in sufficient concentrations and maintain therapeutic efficacy.
Advanced Concepts in Chemical Equilibrium
Beyond the basic principles, several advanced concepts further refine our understanding of chemical equilibrium:
1. Activity and Activity Coefficients:
For highly concentrated solutions, the use of concentrations in the equilibrium constant expression isn't entirely accurate. Activity, a thermodynamic concept, accounts for deviations from ideal behavior. Activity coefficients correct for non-ideal behavior, improving the accuracy of equilibrium calculations.
2. Coupled Equilibria:
Many systems involve multiple simultaneous equilibria. These coupled equilibria are interconnected, and changes in one equilibrium can affect the others. Analyzing such systems requires considering the interaction between multiple equilibrium constants.
3. Heterogeneous Equilibria:
Heterogeneous equilibria involve reactants and products in different phases (e.g., solid, liquid, gas). The concentrations of pure solids and liquids are considered constant, simplifying the equilibrium constant expression.
Conclusion
Chemical equilibrium is a fundamental concept in chemistry with far-reaching implications. Understanding the dynamic nature of equilibrium, the factors influencing it, and its various applications is crucial for anyone working in related fields. From industrial processes to environmental science and biochemistry, mastering the principles of chemical equilibrium is key to solving complex problems and developing innovative solutions. By carefully analyzing the equilibrium constant, applying Le Chatelier's principle, and considering advanced concepts, we can gain a comprehensive understanding of these dynamic systems and harness their power for various applications.
Latest Posts
Latest Posts
-
Select All Structures Produced By Mosses
Mar 28, 2025
-
Discrepancies Between The Actual Self And The Ideal Self
Mar 28, 2025
-
When Does A Chemical Reaction Stop
Mar 28, 2025
-
What Has Definite Shapes And Definite Volumes
Mar 28, 2025
-
How To Subtract Rational Algebraic Expressions
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Properties Of Systems In Chemical Equilibrium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
