What Has Definite Shapes And Definite Volumes
Muz Play
Mar 28, 2025 · 5 min read
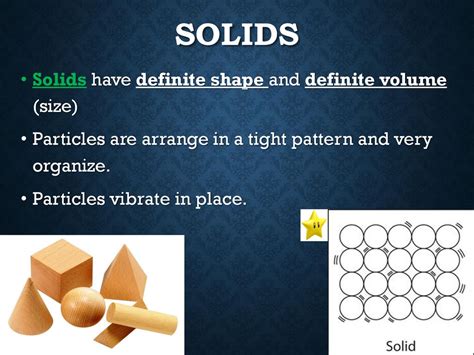
Table of Contents
What Has Definite Shapes and Definite Volumes? Exploring the World of Solids
The world around us is a fascinating tapestry of matter, existing in various states. Understanding the fundamental properties of matter, such as shape and volume, is crucial to comprehending the physical world. This article delves into the characteristics of substances that possess definite shapes and definite volumes, focusing primarily on solids. We'll explore the microscopic structure, macroscopic properties, and real-world examples of these materials, along with a discussion of exceptions and nuances within this category.
Understanding Shape and Volume
Before we dive into specifics, let's clarify the terms "shape" and "volume."
-
Shape: This refers to the external form or outline of an object. A solid's shape is determined by the arrangement of its constituent particles and the forces acting upon them. A cube, for example, has a distinctly different shape from a sphere.
-
Volume: This is the amount of three-dimensional space occupied by an object. It's a measure of how much space a substance takes up. For solids with definite shapes, the volume is directly related to the dimensions of the object.
The Defining Characteristics of Solids
Substances with definite shapes and definite volumes are overwhelmingly solids. This is because of their unique molecular structure. In solids, the constituent particles (atoms, ions, or molecules) are tightly packed together in a highly ordered arrangement. Strong intermolecular forces hold these particles in relatively fixed positions, resulting in:
-
Rigid Structure: The strong attractive forces prevent the particles from moving freely, giving solids their rigidity and resistance to deformation. This inherent resistance to change in shape explains their definite shapes.
-
Incompressibility: The close proximity of particles leaves little empty space. This makes solids largely incompressible, meaning their volume remains relatively constant even under significant pressure. This explains their definite volumes.
Types of Solids and Their Structures
Solids are further classified based on their microscopic structure:
1. Crystalline Solids
Crystalline solids are characterized by a highly ordered, repeating three-dimensional arrangement of particles. This ordered structure extends throughout the entire solid, forming a crystal lattice. Examples include:
-
Ionic solids: Formed by the electrostatic attraction between oppositely charged ions (e.g., sodium chloride, NaCl – common table salt). The strong electrostatic forces contribute to their high melting points and hardness.
-
Covalent network solids: Characterized by a network of covalent bonds extending throughout the entire solid (e.g., diamond, silicon dioxide). These solids are exceptionally hard and have high melting points due to the strong covalent bonds.
-
Metallic solids: Composed of metal atoms held together by metallic bonds (e.g., iron, copper, gold). The delocalized electrons contribute to their high electrical and thermal conductivity and malleability.
-
Molecular solids: Formed by molecules held together by weaker intermolecular forces (e.g., ice, sugar). These solids generally have lower melting points compared to ionic, covalent network, and metallic solids.
2. Amorphous Solids
Unlike crystalline solids, amorphous solids lack a long-range ordered structure. The arrangement of particles is random and disordered. Examples include:
-
Glass: A supercooled liquid with a disordered structure. Its viscosity is extremely high, preventing it from flowing easily at room temperature.
-
Plastics: Polymers with a complex, often tangled, arrangement of molecules. The lack of crystallinity affects their mechanical properties.
-
Rubber: An elastomer with a disordered structure, exhibiting elasticity and flexibility.
Exceptions and Nuances
While the vast majority of solids exhibit definite shapes and definite volumes, there are exceptions and nuances to consider:
-
Deformation: Even rigid solids can undergo deformation under sufficient stress. This can involve changes in shape (e.g., bending, stretching) but usually does not drastically affect the volume. Elastic materials can return to their original shape after the stress is removed, while plastic materials undergo permanent deformation.
-
Temperature Effects: Changes in temperature can affect the volume of a solid through thermal expansion. As temperature increases, the particles gain kinetic energy, leading to a slight increase in volume. However, the shape generally remains relatively consistent unless the temperature change is extreme.
-
Pressure Effects: While solids are relatively incompressible, extremely high pressures can cause a small reduction in volume. This is particularly noticeable in highly compressible solids like some polymers.
-
Fluid-like behavior in some amorphous solids: Amorphous solids can exhibit some liquid-like properties. For example, glass flows very slowly over long periods, although its shape doesn't readily change at room temperature.
Real-World Applications and Examples
The properties of solids with definite shapes and volumes are crucial to countless applications:
-
Construction: Bricks, concrete, steel, and wood are essential building materials that rely on their rigid structure and strength.
-
Manufacturing: Various solid materials are used in the manufacture of countless products, from tools and machinery to consumer electronics and automobiles.
-
Electronics: Semiconductors like silicon are crystalline solids with specific electrical properties that are fundamental to modern electronics.
-
Medicine: Many drugs are solid crystalline materials with specific properties that affect their bioavailability and efficacy.
-
Everyday Objects: Most everyday objects we interact with—from furniture and utensils to books and clothing—are made of solid materials with definite shapes and volumes.
Conclusion: The Importance of Solid State Properties
Solids, with their definite shapes and definite volumes, represent a significant portion of the matter in our world. Their properties, stemming from the strong intermolecular forces and ordered arrangement of particles, are fundamental to understanding the physical world and to countless technological applications. While exceptions and nuances exist, the fundamental principle remains: solids are the quintessential example of materials possessing both definite shape and definite volume. Understanding their microscopic structure provides invaluable insight into their macroscopic behavior and their diverse applications in various fields. Further exploration into the specifics of different solid types and their properties will continue to drive innovation and development across various scientific and engineering disciplines. The seemingly simple concepts of shape and volume are, therefore, gateways to a deeper understanding of the complex world of materials science.
Latest Posts
Latest Posts
-
Moment Of Inertia Of Uniform Rod
Mar 31, 2025
-
Keratin And Collagen Are Examples Of Which Class Of Proteins
Mar 31, 2025
-
Job Order Costing Vs Process Costing
Mar 31, 2025
-
What Is The Chemical Equation For Aerobic Respiration
Mar 31, 2025
-
Equation Of The Tangent Line Implicit Differentiation
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Has Definite Shapes And Definite Volumes . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
