Protons Neutrons And Electrons Of Helium
Muz Play
Mar 30, 2025 · 7 min read
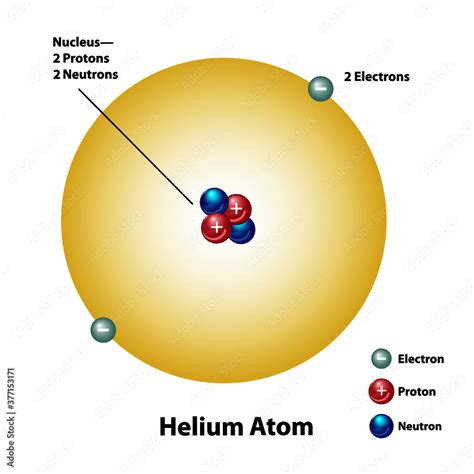
Table of Contents
Delving Deep into Helium: Protons, Neutrons, and Electrons
Helium, the second element on the periodic table, is a fascinating subject for scientific exploration. Its unique properties, stemming from its simple atomic structure, make it crucial in various applications, from cryogenics to medical imaging. Understanding the fundamental building blocks of helium – its protons, neutrons, and electrons – is key to grasping its behavior and significance. This comprehensive article will delve into the intricacies of helium's atomic structure, exploring its isotopes, its role in the universe, and its practical applications.
Helium's Atomic Structure: A Closer Look
Helium's atomic simplicity belies its importance. Its atomic number, 2, indicates that a neutral helium atom possesses two protons in its nucleus. These positively charged particles define helium as helium and distinguish it from all other elements. The number of protons dictates an element's chemical properties.
The Nucleus: Protons and Neutrons
The nucleus, the atom's core, houses not only protons but also neutrons. Neutrons, unlike protons, carry no electrical charge; they are neutral. The number of neutrons can vary, giving rise to different isotopes of helium. The most common isotope, helium-4 (⁴He), contains two neutrons, while the less abundant helium-3 (³He) contains only one neutron. The total number of protons and neutrons is known as the mass number.
The strong nuclear force binds protons and neutrons together within the nucleus, overcoming the electrostatic repulsion between the positively charged protons. This force is incredibly strong at short distances but rapidly weakens with increasing separation. The stability of the helium nucleus is a direct consequence of this strong force. The strong nuclear force is responsible for the stability and integrity of atomic nuclei, and its understanding is vital to comprehending nuclear physics and the behavior of elements.
The Electron Cloud: Electrons and Energy Levels
Surrounding the nucleus is a cloud of negatively charged electrons. These particles are much lighter than protons and neutrons, and their influence is primarily determined by electrostatic attraction to the positively charged nucleus. In a neutral helium atom, there are two electrons, balancing the positive charge of the two protons. These electrons occupy specific energy levels or orbitals, described by quantum mechanics.
For helium, these electrons typically reside in the lowest energy level, the 1s orbital, which can hold a maximum of two electrons. This completely filled electron shell contributes to helium's chemical inertness – its unwillingness to readily form chemical bonds with other atoms. This characteristic is a cornerstone of helium's applications, particularly in situations where non-reactivity is crucial. The stable electron configuration explains why helium is a noble gas, meaning it’s exceptionally unreactive.
Helium Isotopes: Variations on a Theme
While the most common form of helium is helium-4 (⁴He), helium-3 (³He) also exists, albeit in much smaller quantities. These two isotopes differ in their number of neutrons, leading to slight variations in their properties, particularly their mass and nuclear spin.
Helium-4 (⁴He): The Abundant Isotope
Helium-4, with its two protons and two neutrons, constitutes the vast majority of helium found in nature. Its exceptional stability stems from the even number of both protons and neutrons, resulting in a tightly bound nucleus. This stability contributes to its inertness and wide range of applications.
The abundance of helium-4 is a consequence of its formation in stars. Through nuclear fusion, hydrogen atoms combine to form helium, releasing vast amounts of energy in the process. This process is the primary energy source of stars, and it's the main reason helium-4 is so prevalent in the universe.
Helium-3 (³He): The Rarity with Potential
Helium-3, with its single neutron, is much rarer than helium-4. Its lower abundance is mainly due to its less stable nucleus. Its nuclear spin, along with its slightly different mass, imparts unique properties that make it valuable in specific applications, notably in nuclear magnetic resonance (NMR) spectroscopy and as a potential fuel for future fusion reactors.
The potential of helium-3 as a clean and efficient fusion fuel is a subject of ongoing research. Unlike traditional fission reactors, which produce radioactive waste, fusion reactors based on helium-3 could offer a sustainable and environmentally friendly energy source. However, the scarcity of helium-3 presents a significant challenge for its widespread adoption.
Helium's Role in the Universe: From Stars to Earth
Helium plays a significant role in the cosmos, from its origin in stellar nucleosynthesis to its presence in planetary atmospheres. Understanding helium's cosmic journey sheds light on the evolution of stars and the formation of planetary systems.
Stellar Nucleosynthesis: The Birthplace of Helium
The vast majority of helium in the universe was created through stellar nucleosynthesis – the process by which elements are formed within stars. The intense heat and pressure inside stars force hydrogen atoms to fuse together, forming helium and releasing enormous quantities of energy. This process is fundamental to stellar evolution and provides the energy that powers stars for billions of years.
As stars evolve and eventually die, they release helium into interstellar space. This helium then serves as building blocks for the formation of new stars and planetary systems. The abundance of helium in the universe is thus a testament to the continuous process of stellar nucleosynthesis.
Helium on Earth: A Scarce Resource
While abundant in the universe, helium is relatively scarce on Earth. Its low density means it readily escapes Earth's gravitational pull, and most of the helium present on our planet is a product of radioactive decay of heavy elements in the Earth's crust. This radioactive decay produces alpha particles, which are essentially helium nuclei (two protons and two neutrons). These alpha particles then combine with electrons to form helium atoms.
The scarcity of helium on Earth necessitates responsible management and conservation efforts. Helium is a non-renewable resource, and its current rate of consumption is unsustainable. Strategies for helium conservation and recycling are crucial to ensuring its availability for future applications.
Helium's Applications: A Versatile Element
Helium's unique properties, stemming from its atomic structure and inertness, make it indispensable in a wide range of applications. These applications span various scientific, industrial, and medical fields.
Cryogenics: Cooling to Extremely Low Temperatures
Helium's extremely low boiling point (-268.93°C or 4.22 K) makes it ideal for cryogenic applications – the science of achieving extremely low temperatures. Liquid helium is used to cool superconducting magnets in MRI machines, particle accelerators, and other scientific instruments. Its ability to maintain these low temperatures is vital for the operation of these technologies.
The use of liquid helium in cryogenics allows scientists and engineers to explore phenomena that are only observable at extremely low temperatures, pushing the boundaries of scientific discovery.
Leak Detection: Finding Hidden Leaks
Helium's low density and inertness make it an excellent tracer gas for leak detection. By introducing helium into a system and then using a sensitive detector, engineers can pinpoint even the smallest leaks, ensuring the integrity of pressurized systems in various industries.
This application is essential in ensuring safety and efficiency in various industrial settings, ranging from pipelines to spacecraft.
Medical Imaging: MRI and Beyond
Helium's crucial role in medical imaging, particularly in magnetic resonance imaging (MRI), stems from its use in cooling superconducting magnets. These magnets are essential components of MRI machines, producing powerful magnetic fields necessary for creating high-resolution images of the human body.
The use of helium in MRI contributes significantly to the advancement of medical diagnosis and treatment.
Welding and other Industrial Applications
Helium's inertness makes it a valuable shielding gas in various welding processes. It protects the weld from oxidation and other contaminants, ensuring the integrity of the weld joint. Its use extends to other industrial processes requiring an inert atmosphere.
The use of helium in welding and other industrial applications highlights its versatility and importance in ensuring quality and efficiency in various manufacturing processes.
Conclusion: The Significance of Helium
Helium, with its simple yet profound atomic structure, plays a crucial role in various aspects of our world. From its formation in stars to its applications in modern technology, helium’s significance is undeniable. Understanding its protons, neutrons, and electrons provides a deeper appreciation for its unique properties and its indispensable role in science, technology, and medicine. However, the scarcity of helium on Earth necessitates responsible consumption and exploration of sustainable alternatives to ensure its continued availability for future generations. The ongoing research into helium-3 and its potential as a clean energy source further emphasizes the continuing scientific importance and potential of this remarkable element.
Latest Posts
Latest Posts
-
How To Calculate Standard Free Energy Change
Apr 01, 2025
-
Root Mean Square Velocity Of Gas
Apr 01, 2025
-
Where Is The Respiratory Center Located In The Brain
Apr 01, 2025
-
What Is The Difference Between Cellular Respiration And Fermentation
Apr 01, 2025
-
What Are Two Functional Groups Found In Amino Acids
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Protons Neutrons And Electrons Of Helium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
