Reaction Of Ester With Lithium Aluminium Hydride
Muz Play
Mar 18, 2025 · 5 min read
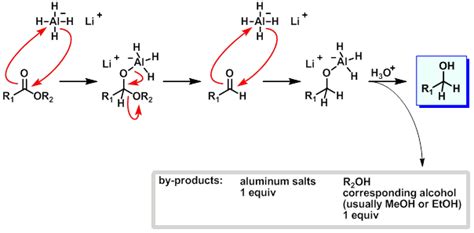
Table of Contents
The Reaction of Esters with Lithium Aluminum Hydride: A Comprehensive Guide
The reduction of esters using lithium aluminum hydride (LiAlH₄) is a cornerstone reaction in organic chemistry, offering a powerful and versatile method for synthesizing primary alcohols. This reaction's widespread use stems from its high efficiency and the relative accessibility of both esters and LiAlH₄. This comprehensive guide will delve into the mechanism, reaction conditions, applications, and limitations of this crucial transformation.
Understanding Lithium Aluminum Hydride (LiAlH₄)
Lithium aluminum hydride is a powerful reducing agent, often referred to as LAH. Its potency arises from the presence of the hydride ion (H⁻), a potent nucleophile. The aluminum atom, with its relatively low electronegativity, readily donates electron density to the hydride, making it even more reactive. This characteristic makes LAH capable of reducing a variety of functional groups, including esters, ketones, aldehydes, and nitriles. However, its reactivity also necessitates careful handling and specific reaction conditions to avoid unwanted side reactions. LAH is typically used as a suspension in anhydrous diethyl ether or tetrahydrofuran (THF), solvents crucial for its stability and reactivity.
The Mechanism of Ester Reduction with LiAlH₄
The reduction of an ester with LiAlH₄ proceeds through a four-step mechanism:
Step 1: Nucleophilic Attack by Hydride
The reaction initiates with the nucleophilic attack of the hydride ion (H⁻) from LiAlH₄ on the carbonyl carbon of the ester. This attack leads to the formation of a tetrahedral intermediate. The carbonyl carbon, initially electrophilic, becomes temporarily saturated, resulting in the breakage of the π bond.
Step 2: Formation of an Alkoxide Intermediate
The tetrahedral intermediate is unstable and readily collapses. This collapse involves the expulsion of the alkoxide group (RO⁻), leaving behind an aldehyde intermediate. The alkoxide ion is stabilized by its interaction with the aluminum atom in the LiAlH₄ complex.
Step 3: Second Nucleophilic Attack
The aldehyde intermediate, also electrophilic, is rapidly attacked by another hydride ion from LiAlH₄. This second nucleophilic attack forms a second tetrahedral intermediate.
Step 4: Hydrolysis and Alcohol Formation
The final step involves hydrolysis. The addition of water or a dilute acid (like sulfuric acid or hydrochloric acid) protonates the alkoxide ion, releasing the primary alcohol and aluminum hydroxide. This hydrolysis step is essential for liberating the alcohol product from the aluminum complex.
Overall Reaction:
RCOOR' + 4[H] → RCH₂OH + R'OH
Where [H] represents a hydride equivalent from LiAlH₄.
Reaction Conditions and Considerations
The success of the ester reduction with LiAlH₄ hinges on meticulous control of reaction conditions. Several factors play crucial roles:
- Solvent: Anhydrous diethyl ether or THF are preferred solvents due to their ability to dissolve LiAlH₄ and stabilize the reaction intermediates. The presence of even trace amounts of water can deactivate LAH, significantly reducing the yield.
- Temperature: The reaction is typically carried out at low to moderate temperatures (0°C to reflux), depending on the specific ester and desired reaction rate. Higher temperatures can promote side reactions.
- Stoichiometry: A stoichiometric excess of LiAlH₄ (typically 4 equivalents or more) is often used to ensure complete reduction. This excess compensates for potential side reactions and incomplete reduction.
- Workup Procedure: The workup procedure is crucial for safely isolating the alcohol product. The addition of water or dilute acid should be carried out cautiously and slowly to avoid vigorous reactions and potential hazards. The workup procedure often involves quenching the excess LiAlH₄ with a proton source like dilute acid, followed by extraction, washing, and drying to isolate the purified product.
Applications and Examples of Ester Reduction
The reduction of esters using LiAlH₄ finds extensive applications in organic synthesis, enabling the preparation of a wide range of primary alcohols. Here are some examples:
- Synthesis of Pharmaceuticals: Many pharmaceutical molecules contain alcohol functionalities. LiAlH₄ reduction of esters serves as a key step in their synthesis.
- Preparation of Fragrance and Flavor Compounds: Several fragrances and flavor compounds possess alcohol groups. The conversion of esters to alcohols using LiAlH₄ enables the preparation of these important compounds.
- Synthesis of Natural Products: Many natural products incorporate alcohol functionalities. LiAlH₄-mediated reduction of esters plays a critical role in synthesizing these complex molecules.
- Polymer Chemistry: The synthesis of certain polymers involves the reduction of ester groups. LAH's reducing power is useful in this area.
Specific Examples:
-
Reduction of Ethyl Acetate: Ethyl acetate, upon reaction with LiAlH₄, yields ethanol.
-
Reduction of Methyl Benzoate: Methyl benzoate, when reacted with LiAlH₄, gives benzyl alcohol.
-
Reduction of Complex Esters: LiAlH₄ efficiently reduces more complex esters, such as those containing other functional groups, providing a versatile approach to alcohol synthesis. However, careful consideration of the presence of other reducible functional groups is necessary.
Limitations and Considerations
Despite its power, LiAlH₄ reduction of esters has some limitations:
- Sensitivity to Moisture: LAH is extremely sensitive to moisture and reacts violently with water. Strict anhydrous conditions are essential to prevent decomposition and side reactions.
- Reactivity with Other Functional Groups: LiAlH₄ is a powerful reducing agent capable of reducing other functional groups besides esters. The presence of other reducible functionalities necessitates careful reaction planning and potentially alternative reducing agents. For instance, halides, nitro groups, and some other functional groups are also prone to reduction by LiAlH₄.
- Safety Precautions: LAH is a strong base and reacts vigorously with water, producing flammable hydrogen gas. Appropriate safety precautions, including the use of appropriate personal protective equipment (PPE) and controlled handling procedures, are essential when working with LAH.
Alternative Reducing Agents
While LiAlH₄ is a powerful and widely used reagent for ester reduction, other reducing agents can be employed, offering alternative advantages in specific situations:
- Diborane (B₂H₆): Diborane is a milder reducing agent compared to LAH, offering greater selectivity in the reduction of esters in the presence of other functional groups.
- Sodium Borohydride (NaBH₄): Sodium borohydride is a much milder reducing agent than LAH. It will not reduce esters.
Conclusion
The reduction of esters with lithium aluminum hydride is a fundamental reaction in organic chemistry, providing a robust and versatile method for synthesizing primary alcohols. The mechanism, reaction conditions, applications, and limitations have been discussed in detail. Understanding these aspects is crucial for successfully implementing this reaction in various synthetic strategies. While LiAlH₄ offers significant advantages, careful consideration of its sensitivity to moisture, potential reactivity with other functional groups, and safety precautions is paramount for achieving high yields and safe experimentation. The choice of reducing agent should always be carefully considered based on the specific substrate and desired outcome. The detailed understanding outlined in this guide empowers chemists to effectively leverage this powerful tool in organic synthesis.
Latest Posts
Latest Posts
-
How Do You Convert Moles To Volume
Mar 19, 2025
-
The Horizontal Columns On The Periodic Table Are Called
Mar 19, 2025
-
How To Find Derivative Of Limit
Mar 19, 2025
-
Proof Of The Parallel Axis Theorem
Mar 19, 2025
-
What Is Primary And Secondary Growth In Plants
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Reaction Of Ester With Lithium Aluminium Hydride . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
