Reduction Of Carboxylic Acid To Aldehyde
Muz Play
Mar 19, 2025 · 6 min read
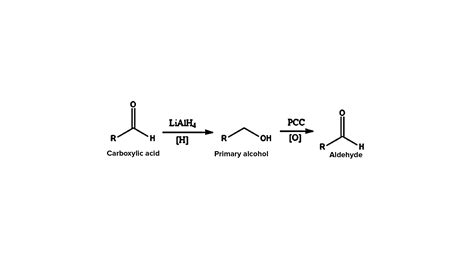
Table of Contents
Reduction of Carboxylic Acids to Aldehydes: A Comprehensive Guide
The reduction of carboxylic acids to aldehydes is a challenging transformation in organic chemistry. Unlike the straightforward reduction of ketones and aldehydes to alcohols, directly converting a carboxylic acid to an aldehyde requires careful control to prevent over-reduction to the alcohol. This comprehensive guide delves into the intricacies of this reaction, exploring various methods, their mechanisms, advantages, disadvantages, and applications.
The Challenge: Preventing Over-Reduction
The core difficulty in reducing carboxylic acids to aldehydes lies in the aldehyde's inherent reactivity. Aldehydes are more easily reduced than carboxylic acids, meaning that once formed, they readily undergo further reduction to the corresponding alcohol. This necessitates the use of carefully chosen reagents and reaction conditions to selectively achieve the aldehyde product. A delicate balance between reactivity and selectivity is crucial.
Methods for Reducing Carboxylic Acids to Aldehydes
Several methods have been developed to achieve this selective reduction, each with its own strengths and limitations. These methods can be broadly categorized based on the reducing agent employed.
1. Using Reducing Agents: A Detailed Look
a) Lithium Aluminum Hydride (LAH) Modifications: While LAH itself typically reduces carboxylic acids all the way to alcohols, modified approaches can offer some control. The use of LAH in conjunction with specific reaction conditions, like low temperatures and careful control of stoichiometry, might give a small amount of aldehyde, but it's far from a reliable method for selective aldehyde synthesis. The primary product will almost always be the alcohol.
b) Boron-Based Reducing Agents: These reagents have shown some promise in achieving selectivity. Examples include diisobutylaluminum hydride (DIBAL-H) and 9-borabicyclo[3.3.1]nonane (9-BBN). These reagents tend to be less reactive than LAH, allowing for better control over the reduction process.
i) DIBAL-H (Diisobutylaluminum Hydride): This is perhaps the most commonly used reagent for the reduction of carboxylic acids to aldehydes. DIBAL-H is a powerful reducing agent, but its reactivity can be controlled by adjusting the reaction temperature and the stoichiometry of the reagents. The reaction is typically carried out at low temperatures (e.g., -78°C) in an aprotic solvent like toluene or dichloromethane. The reaction mechanism involves the coordination of DIBAL-H to the carbonyl oxygen, followed by hydride transfer and subsequent workup to yield the aldehyde.
Advantages of DIBAL-H:
- Relatively high selectivity for aldehyde formation.
- Widely applicable to a range of carboxylic acids.
Disadvantages of DIBAL-H:
- Requires low temperatures, which can be inconvenient.
- Can be sensitive to moisture and air.
- Stoichiometric reagent, making it less atom-economical than catalytic methods.
ii) Other Boron Reagents: Other boron-based reducing agents are also being explored. The development of new, more selective, and efficient boron reagents is an active area of research in organic synthesis. The aim is often to improve the yield, reduce the need for harsh reaction conditions, and expand the scope of substrates that can be effectively reduced.
2. Electrochemical Reduction: A Green Approach
Electrochemical methods represent a greener alternative to traditional chemical reduction. These methods employ an electrode to facilitate the reduction process, often utilizing a sacrificial anode. By carefully controlling the applied potential, it's possible to achieve selective reduction to the aldehyde. This approach is environmentally friendlier due to the avoidance of stoichiometric reducing agents. However, electrochemical methods often require specialized equipment and optimized reaction conditions, making them less widely accessible than chemical reductions.
3. Enzyme-Catalyzed Reduction: Biocatalysis in Action
Enzymes offer a highly selective and environmentally benign approach to organic synthesis. Certain enzymes, particularly those found in microorganisms, can catalyze the reduction of carboxylic acids to aldehydes with remarkable selectivity. The use of enzymes often requires specific reaction conditions and careful optimization for each substrate. While offering exceptional selectivity, the broader applicability and scalability of enzyme-catalyzed reductions are still under development.
Reaction Mechanisms: A Closer Look
The mechanisms of reduction vary depending on the reducing agent used. However, many involve a common initial step: nucleophilic attack of the hydride ion (or a similar species) on the carbonyl carbon of the carboxylic acid. The specifics, however, differ significantly.
DIBAL-H Mechanism: The mechanism involves the stepwise addition of two hydrides. The first hydride addition leads to the formation of an alkoxy aluminum intermediate. A second hydride transfer, followed by hydrolysis, yields the aldehyde. The low temperature is crucial to prevent over-reduction.
Electrochemical Reduction Mechanism: The exact mechanism is complex and depends on the specific reaction conditions and the electrode material. In general, it involves electron transfer to the carboxylic acid, followed by protonation and further reduction steps, eventually leading to aldehyde formation.
Protecting Groups: Strategies for Selective Reduction
Protecting group strategies can be employed to prevent over-reduction. A common approach is to convert the carboxylic acid into a less reactive derivative, such as an ester or an activated ester. The less reactive derivative is then reduced using a milder reducing agent, followed by deprotection to obtain the aldehyde. While this is a two-step process, it improves selectivity. The choice of protecting group depends on the specific substrate and the desired reactivity.
Applications of Carboxylic Acid Reduction to Aldehydes
The ability to selectively reduce carboxylic acids to aldehydes is crucial in many areas of organic synthesis and chemical manufacturing.
- Synthesis of Fragrances and Flavors: Many naturally occurring aldehydes are important components of fragrances and flavors. The selective reduction of carboxylic acids is a key step in the synthesis of these valuable compounds.
- Pharmaceutical Chemistry: Aldehydes are frequently found as key structural motifs in pharmaceuticals. The selective reduction of carboxylic acids is often a crucial step in the synthesis of these medicinal agents.
- Materials Science: Aldehydes can be used as building blocks for the synthesis of various polymeric materials. The controlled synthesis of aldehydes through carboxylic acid reduction is essential in materials science.
- Organic Synthesis as Intermediates: Aldehydes serve as versatile intermediates in numerous organic transformations. Their ability to undergo further reactions, like oxidation, reduction, nucleophilic addition, and condensation, makes them important building blocks in complex molecule synthesis.
Conclusion: Navigating the Complexities
The reduction of carboxylic acids to aldehydes is a significant challenge in organic chemistry, requiring precise control over reaction conditions and reagent choice. While DIBAL-H remains a dominant method, continued research into novel reducing agents, including boron-based reagents, electrochemical methods, and biocatalysis, promises to deliver even more efficient, selective, and environmentally benign approaches. The careful selection of the method, along with a deep understanding of reaction mechanisms and the use of protecting groups when necessary, is paramount in achieving successful and high-yielding transformations. The applications of this crucial reaction are widespread and continue to expand across diverse scientific disciplines. Future developments in this area will undoubtedly lead to even more sophisticated and practical methods for the selective synthesis of aldehydes from carboxylic acids.
Latest Posts
Latest Posts
-
Using Inverse Matrix To Solve System Of Linear Equations
Mar 19, 2025
-
Is H2o An Acid Or Base
Mar 19, 2025
-
What Are The Membrane Bound Organelles
Mar 19, 2025
-
What Does A Penetration Pricing Demand Curve Look Like
Mar 19, 2025
-
How To Calculate Heat Of Reaction In Kj Mol
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Reduction Of Carboxylic Acid To Aldehyde . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
