Is H2o An Acid Or Base
Muz Play
Mar 19, 2025 · 6 min read
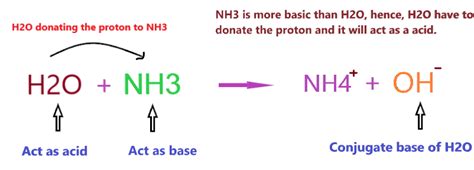
Table of Contents
Is H₂O an Acid or a Base? Understanding the Amphoteric Nature of Water
Water, the elixir of life, is a ubiquitous substance crucial for all known forms of life. Its chemical formula, H₂O, is simple, yet its behavior as an acid or base is surprisingly nuanced. While often perceived as neutral, water exhibits amphoteric properties, meaning it can act as both an acid and a base depending on the circumstances. This article delves into the intricacies of water's acidic and basic behavior, exploring its self-ionization, the pH scale, and its role in various chemical reactions.
The Brønsted-Lowry Definition: Acids and Bases
To understand water's behavior, we need to define acids and bases. The Brønsted-Lowry theory, a widely accepted model, defines an acid as a substance that donates a proton (H⁺ ion) and a base as a substance that accepts a proton.
Water as a Proton Donor (Acid):
Water can act as an acid by donating a proton to a suitable base. Consider the reaction between water and ammonia (NH₃):
H₂O + NH₃ ⇌ NH₄⁺ + OH⁻
In this reaction, water donates a proton (H⁺) to ammonia, forming the ammonium ion (NH₄⁺) and the hydroxide ion (OH⁻). Water acts as an acid because it donates a proton.
Water as a Proton Acceptor (Base):
Conversely, water can also act as a base by accepting a proton from a suitable acid. Consider the reaction between water and hydrogen chloride (HCl):
H₂O + HCl ⇌ H₃O⁺ + Cl⁻
In this reaction, water accepts a proton (H⁺) from hydrochloric acid, forming the hydronium ion (H₃O⁺) and the chloride ion (Cl⁻). Water acts as a base because it accepts a proton.
Self-Ionization of Water: The Key to its Amphoteric Nature
The amphoteric nature of water is most clearly demonstrated by its ability to undergo self-ionization. This process involves two water molecules reacting to form a hydronium ion (H₃O⁺) and a hydroxide ion (OH⁻):
2H₂O ⇌ H₃O⁺ + OH⁻
This equilibrium reaction is crucial because it establishes the concentration of H₃O⁺ and OH⁻ ions in pure water. At 25°C, the concentration of both ions is 1.0 × 10⁻⁷ M. This equilibrium constant, Kw (the ion product constant of water), is given by:
Kw = [H₃O⁺][OH⁻] = 1.0 × 10⁻¹⁴ at 25°C
This equation shows that the product of the concentrations of hydronium and hydroxide ions is always constant at a given temperature.
The Significance of Kw:
The value of Kw is fundamental in understanding the pH scale and the acidity or basicity of aqueous solutions. It shows that even in pure water, there is a small but significant concentration of both H₃O⁺ and OH⁻ ions. This self-ionization is responsible for water's ability to act as both an acid and a base.
The pH Scale: Measuring Acidity and Basicity
The pH scale is a logarithmic scale used to express the acidity or basicity of a solution. It ranges from 0 to 14, with 7 representing neutrality.
- pH < 7: Acidic solution – [H₃O⁺] > [OH⁻]
- pH = 7: Neutral solution – [H₃O⁺] = [OH⁻]
- pH > 7: Basic solution – [H₃O⁺] < [OH⁻]
The pH is calculated using the following equation:
pH = -log₁₀[H₃O⁺]
Pure water at 25°C has a pH of 7, indicating its neutral nature. However, it's crucial to remember that this neutrality is a consequence of the equal concentrations of H₃O⁺ and OH⁻ ions resulting from self-ionization. The presence of these ions is what allows water to act as both an acid and a base in reactions with other substances.
Water's Role in Chemical Reactions: A Versatile Solvent
Water's amphoteric nature plays a vital role in its ability to act as a solvent in countless chemical reactions. Its ability to both donate and accept protons facilitates the dissolution and reaction of many ionic and polar substances. For example, in the dissolution of salts like NaCl:
NaCl(s) + H₂O(l) → Na⁺(aq) + Cl⁻(aq)
Water molecules surround the Na⁺ and Cl⁻ ions, stabilizing them in solution. This solvation process relies on the polar nature of water and its ability to interact with charged species.
Water in Acid-Base Reactions:
Water's amphoteric behavior is central to many acid-base reactions. It can act as a solvent, facilitating the reaction between an acid and a base, and also directly participate as a reactant, either donating or accepting protons. This is exemplified in neutralization reactions, where an acid and a base react to form water and a salt.
For instance, the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH):
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
In this reaction, the H⁺ from HCl reacts with the OH⁻ from NaOH to form water, showcasing water's role as a product of a neutralization reaction.
Factors Affecting Water's Acidity and Basicity
While pure water is neutral at 25°C, several factors can influence its apparent acidity or basicity:
-
Temperature: The Kw value, and thus the pH of pure water, varies with temperature. At higher temperatures, the self-ionization of water increases, leading to a slightly lower pH (slightly more acidic).
-
Dissolved Substances: The presence of dissolved substances, particularly acids or bases, significantly affects the pH of water. Adding an acid decreases the pH, while adding a base increases the pH. Even seemingly pure water often contains dissolved gases like carbon dioxide (CO₂), which can react with water to form carbonic acid (H₂CO₃), lowering the pH.
-
Pressure: Pressure also plays a subtle role in altering the equilibrium of water self-ionization, though the effect is generally less significant than temperature.
Conclusion: Water – More Than Just a Neutral Solvent
In summary, while often considered neutral, water exhibits a remarkable amphoteric nature, readily acting as both an acid and a base. Its self-ionization, characterized by the equilibrium between H₃O⁺ and OH⁻ ions, is the foundation of its dual behavior. The pH scale provides a quantitative measure of the acidity or basicity of aqueous solutions, highlighting water's central role in determining the overall chemical environment. Understanding water's amphoteric nature is crucial for comprehending various chemical reactions, its solvent properties, and its indispensable role in numerous biological processes. It is far more than a simple neutral solvent; it is a dynamic and versatile participant in the chemical world. Further exploration into the properties of water reveals a fascinating complexity underlying its simple chemical formula. Its seemingly straightforward nature belies a deeper scientific understanding of its behavior, one that continues to captivate scientists and researchers alike. The ongoing study of water's properties and its interactions with other substances continues to unveil new insights into the intricate workings of the natural world.
Latest Posts
Latest Posts
-
How Does Osmosis Affect Animal Cells
Mar 19, 2025
-
Algebra 1 Factor The Common Factor Out Of Each Expression
Mar 19, 2025
-
Weak Acid And Weak Base Ph
Mar 19, 2025
-
Is There A Keyboard Corelation To Find Periodic Table Fonts
Mar 19, 2025
-
The Principal Owes The Agent The Duties Of
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Is H2o An Acid Or Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
