Relationship Between Temperature And Kinetic Energy
Muz Play
Mar 31, 2025 · 6 min read
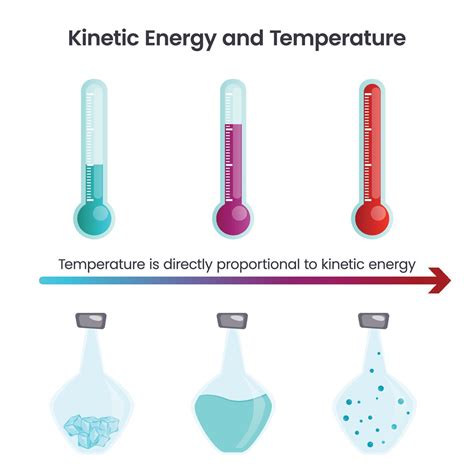
Table of Contents
The Intimate Dance of Temperature and Kinetic Energy: A Deep Dive
The world around us is a symphony of motion, a constant hum of atoms and molecules vibrating, rotating, and translating. This ceaseless movement is the essence of kinetic energy, and its relationship with temperature is fundamental to understanding the physical world. From the melting of ice to the boiling of water, from the expansion of gases to the contraction of solids, temperature acts as a conductor, orchestrating this molecular ballet. This article delves deep into the intricate relationship between temperature and kinetic energy, exploring the underlying principles, diverse applications, and nuances of this pivotal concept in physics.
Understanding Kinetic Energy: The Energy of Motion
Before exploring the connection between temperature and kinetic energy, let's firmly grasp the concept of kinetic energy itself. Simply put, kinetic energy is the energy possessed by an object due to its motion. The faster an object moves, the greater its kinetic energy. This applies not just to macroscopic objects like cars and planets, but also to microscopic particles like atoms and molecules. The kinetic energy of a particle depends on its mass (m) and velocity (v), described by the equation:
KE = ½mv²
This seemingly simple equation holds immense significance, as it forms the bedrock for understanding many physical phenomena, especially when applied to the microscopic world of atoms and molecules.
Kinetic Energy at the Molecular Level: The Heart of the Matter
At the atomic and molecular level, kinetic energy manifests in various forms:
-
Translational Kinetic Energy: This refers to the energy associated with the movement of a particle from one location to another. Imagine a molecule zipping across a container – this is translational kinetic energy in action.
-
Rotational Kinetic Energy: Molecules, unlike single atoms, can also rotate. The energy associated with this spinning motion is rotational kinetic energy. The complexity of the molecule influences its rotational kinetic energy; more complex molecules with multiple bonds have greater capacity for rotational motion.
-
Vibrational Kinetic Energy: Atoms within a molecule are constantly vibrating, oscillating back and forth along their bonds. This vibrational motion contributes significantly to the total kinetic energy of the molecule, particularly at higher temperatures.
The sum of translational, rotational, and vibrational kinetic energies constitutes the total kinetic energy of a molecule. It’s this total kinetic energy that is directly linked to temperature.
Temperature: A Measure of Average Kinetic Energy
Temperature is not merely a measure of "hotness" or "coldness"; it's a precise, quantitative measure of the average kinetic energy of the particles within a substance. This means that a higher temperature signifies a greater average kinetic energy of the constituent particles. Conversely, a lower temperature indicates a lower average kinetic energy.
It's crucial to emphasize the word "average." Within a substance at a given temperature, individual particles possess a range of kinetic energies; some move faster, others slower. Temperature, however, provides a statistical representation of this average kinetic energy. This average kinetic energy is directly proportional to the absolute temperature (measured in Kelvin).
The Kelvin Scale: The Absolute Temperature Scale
The Kelvin scale is unique because it begins at absolute zero (0 K), which represents the theoretical point where all molecular motion ceases. In Celsius and Fahrenheit scales, zero is an arbitrary point, but zero Kelvin is the point of zero kinetic energy. This makes the Kelvin scale ideal for representing the relationship between temperature and kinetic energy. The conversion from Celsius (°C) to Kelvin (K) is straightforward:
K = °C + 273.15
The Impact of Temperature Changes on Kinetic Energy
Changes in temperature directly influence the kinetic energy of particles. Let's explore how:
-
Temperature Increase: As temperature rises, the average kinetic energy of the particles increases proportionally. This leads to faster translational, rotational, and vibrational motions. This increased kinetic energy manifests in various observable effects, such as expansion of materials and increased reaction rates.
-
Temperature Decrease: When the temperature decreases, the average kinetic energy of the particles diminishes. Particles move slower, resulting in contraction of materials and slower reaction rates. At extremely low temperatures (approaching absolute zero), molecular motion becomes minimal.
Phase Transitions: A Kinetic Energy Perspective
Phase transitions – changes in the state of matter (solid, liquid, gas, plasma) – are spectacular examples of the temperature-kinetic energy relationship.
-
Melting: As a solid is heated, the kinetic energy of its particles increases until they overcome the attractive forces holding them in a fixed lattice structure. This results in the transition from solid to liquid.
-
Boiling: Further heating increases kinetic energy to the point where particles gain enough energy to overcome the intermolecular forces holding them together entirely, transitioning from liquid to gas.
-
Sublimation: In some cases, substances can transition directly from solid to gas (sublimation) or gas to solid (deposition), bypassing the liquid phase. These transitions also involve significant changes in the average kinetic energy of the particles.
Applications of the Temperature-Kinetic Energy Relationship
The relationship between temperature and kinetic energy isn't merely an academic concept; it has far-reaching practical applications across diverse fields:
-
Chemistry: Reaction rates are highly dependent on temperature. Higher temperatures increase the kinetic energy of reactant molecules, leading to more frequent and energetic collisions, thus accelerating the reaction.
-
Engineering: Understanding thermal expansion and contraction is crucial in designing structures, machines, and transportation systems. This knowledge is essential for designing materials that can withstand extreme temperature variations without significant structural changes.
-
Meteorology: Temperature differences drive weather patterns, from gentle breezes to violent storms. The kinetic energy of air molecules is the driving force behind atmospheric circulation, precipitation, and other weather phenomena.
-
Biology: Biological processes are heavily influenced by temperature. Enzymes, the catalysts of life, function optimally within specific temperature ranges. Extremes of temperature can denature enzymes and disrupt cellular processes, emphasizing the critical role of temperature in maintaining life.
Beyond the Average: A Deeper Look at Molecular Motion
While temperature provides the average kinetic energy, it's important to acknowledge the distribution of kinetic energies within a system. This is described by the Maxwell-Boltzmann distribution, which shows the probability of a molecule possessing a particular kinetic energy at a given temperature. The curve shows that at any temperature, there is a range of kinetic energies present, with the average aligning with the temperature.
Understanding this distribution is crucial for comprehending phenomena like evaporation. Even at temperatures below the boiling point, some molecules possess enough kinetic energy to overcome intermolecular forces and escape into the gaseous phase. This is why evaporation occurs even at room temperature.
Conclusion: A Fundamental Interplay
The relationship between temperature and kinetic energy is a cornerstone of physics and chemistry, explaining a vast array of natural phenomena and underpinning numerous technological advancements. Understanding this intricate dance of motion and energy is essential for comprehending the world around us, from the smallest molecules to the largest celestial bodies. Whether it’s designing a more efficient engine, predicting weather patterns, or understanding biological processes, mastering this concept unlocks a deeper appreciation for the dynamic world we inhabit. From the microscopic world of atoms to the macroscopic world of everyday objects, temperature and kinetic energy are intrinsically linked, a fundamental interplay shaping the universe we experience.
Latest Posts
Latest Posts
-
Builds A New Dna Strand By Adding Complementary Bases
Apr 01, 2025
-
Which Of The Following Situations Will Lead To Natural Selection
Apr 01, 2025
-
How Does The Nucleus And Ribosomes Work Together
Apr 01, 2025
-
Having A Single Set Of Unpaired Chromosomes
Apr 01, 2025
-
How To Calculate Current In A Series Circuit
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Relationship Between Temperature And Kinetic Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
