Rf Value In Thin Layer Chromatography
Muz Play
Mar 20, 2025 · 7 min read
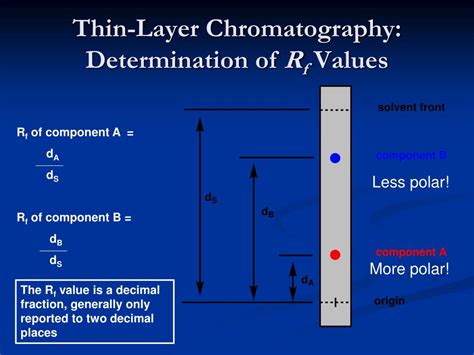
Table of Contents
Understanding Rf Values in Thin Layer Chromatography: A Comprehensive Guide
Thin layer chromatography (TLC) is a widely used analytical technique in chemistry and biochemistry for separating components of a mixture. The technique relies on the differential partitioning of compounds between a stationary phase (typically a silica gel or alumina plate) and a mobile phase (a solvent or solvent mixture). The result of this separation is visualized as distinct spots, each representing a different component of the initial mixture. Crucially, the position of these spots is quantified using the retention factor (Rf), a key parameter for identifying and characterizing compounds. This article provides a comprehensive overview of Rf values in TLC, encompassing its calculation, interpretation, and significance in various applications.
What is Rf Value?
The Rf value, or retention factor, is a dimensionless number that represents the ratio of the distance traveled by a compound to the distance traveled by the solvent front in TLC. It's a critical metric because it's characteristic of a specific compound under specific chromatographic conditions. This means that a particular compound will consistently exhibit the same Rf value (or a very similar one) when the same stationary and mobile phases are employed under controlled conditions.
Formula for calculating Rf:
Rf = (Distance traveled by the compound) / (Distance traveled by the solvent front)
Both distances are measured from the origin (where the sample was spotted). The Rf value is always less than 1 because the compound will always travel a shorter distance than the solvent front. An Rf value of 0 indicates that the compound did not move from the origin, while an Rf value close to 1 suggests that the compound has strong affinity for the mobile phase and travels almost as far as the solvent front.
Factors Affecting Rf Values
Several factors can influence the Rf value obtained in a TLC experiment. Understanding these factors is crucial for accurate interpretation and reproducibility of results. These include:
1. The Stationary Phase:
The type of stationary phase significantly impacts the Rf value. Silica gel, a common stationary phase, is polar, meaning it interacts strongly with polar compounds. Nonpolar compounds will travel further up the plate, resulting in higher Rf values. Alumina, another stationary phase, can also be used and offers different interactions with various compounds. The particle size and thickness of the stationary phase layer also play a role; variations here can lead to variations in Rf values.
2. The Mobile Phase:
The composition of the mobile phase (the solvent system) is arguably the most influential factor determining Rf values. A more polar mobile phase will carry more polar compounds further up the plate, resulting in higher Rf values for those compounds. Conversely, a less polar mobile phase will retain polar compounds at the origin, leading to lower Rf values. The solvent's polarity, as well as the ratio of different solvents in a mixed mobile phase, greatly influences the separation and Rf values.
3. Temperature:
Temperature affects the solvent's viscosity and its interaction with both the stationary and the mobile phase. Changes in temperature can subtly change the Rf values. Consistent temperature control during the TLC process is essential for reproducibility.
4. Saturation of the Chamber:
The chamber where the TLC plate is developed should ideally be saturated with the mobile phase vapor. This saturation ensures uniform conditions and prevents evaporation of the solvent during the development process. Unsaturated chambers can result in uneven solvent fronts and inaccurate Rf values.
5. Sample Loading:
Overloading the sample on the TLC plate can cause streaking or tailing, making accurate measurement of the distance traveled difficult and leading to inaccurate Rf values. Careful spotting of minimal sample volumes is crucial.
6. Quality of TLC Plate:
The quality of the TLC plate itself, including the uniformity of the stationary phase layer, can influence the Rf values. Consistent use of high-quality plates from a reliable supplier is recommended.
Interpreting Rf Values: A Practical Guide
Interpreting Rf values involves more than just calculating the numerical value. It's a process of comparing Rf values obtained for different compounds under identical chromatographic conditions. This allows for the identification of compounds by comparison with known standards.
-
Comparison with Standards: Running known standards alongside the unknown mixture is essential for identification. If an unknown compound exhibits the same Rf value as a known standard under the same conditions, it suggests they might be the same compound. This, however, is not definitive proof; further tests might be needed for confirmation.
-
Effect of Mobile Phase Variations: By systematically altering the composition of the mobile phase (e.g., changing the ratio of solvents), one can fine-tune the separation and obtain optimal Rf values for improved identification. This is particularly helpful when compounds have very similar Rf values under initial conditions.
-
Limitations of Rf Values: While Rf values are useful for preliminary identification, they are not absolute identifiers. Identical Rf values for two different compounds are possible, especially with limited mobile phase optimization. Other techniques, such as spectroscopic methods (NMR, IR, Mass Spectrometry), are usually required for definitive identification.
Applications of Rf Values
Rf values find wide applications in various fields, including:
1. Natural Product Chemistry:
TLC is routinely employed to monitor the progress of extraction and isolation of natural products. The Rf values help track the separation and purification of compounds from plant extracts, providing valuable insights into the composition of the extract.
2. Pharmaceutical Analysis:
TLC is used for quality control and analysis of pharmaceutical formulations. Rf values help to determine the purity of drugs and ensure the absence of impurities or degradation products.
3. Forensic Science:
TLC is a valuable tool in forensic science for analyzing drug samples, inks, dyes, and other materials found at crime scenes. Rf values aid in the identification of controlled substances and can provide crucial evidence in investigations.
4. Environmental Monitoring:
TLC finds applications in environmental analysis for detecting and identifying pollutants in water, soil, and air samples. Rf values can help characterize and quantify the pollutants.
Advanced TLC Techniques and Rf Values
Several advanced TLC techniques offer enhanced resolution and provide more accurate Rf values. These include:
-
High-Performance Thin Layer Chromatography (HPTLC): HPTLC uses plates with smaller particle sizes, providing higher resolution and sharper bands, leading to more precise Rf value measurements.
-
Two-Dimensional TLC: This technique involves developing the plate in two different solvent systems, sequentially, at 90-degree angles. It's particularly useful for resolving complex mixtures that cannot be adequately separated with a single solvent system. In two-dimensional TLC, two Rf values are generated for each compound (one for each dimension).
-
Preparative TLC: This is a scaled-up version of TLC used for isolating and purifying compounds. While primarily focused on purification, the Rf values of the collected fractions can help identify the isolated compounds.
Conclusion: Rf Values and the Future of TLC
The Rf value is a fundamental parameter in thin layer chromatography that provides valuable information about the components of a mixture. Its determination, along with careful consideration of factors affecting it, allows for compound identification and monitoring during chemical processes. While limitations exist, especially in its standalone use for definitive identification, TLC remains a powerful, cost-effective, and versatile analytical technique in a wide range of scientific fields. The development of advanced TLC techniques, like HPTLC, continues to enhance the accuracy and applications of this classic method, ensuring its relevance in modern analytical chemistry. The simple calculation of the Rf value serves as a gateway to understanding the complex world of compound separation and identification, making it an essential skill for any chemist or scientist working with mixtures. Furthermore, the continued development of software and digital imaging techniques will further streamline the process of obtaining and interpreting Rf values, making TLC even more accessible and efficient in the future.
Latest Posts
Latest Posts
-
Law Of Segregation Vs Law Of Independent Assortment
Mar 20, 2025
-
Are Substances With A High Melting Point Soluble
Mar 20, 2025
-
Members Of The Kingdom Fungi Are Photosynthetic
Mar 20, 2025
-
Organic Chemistry Substitution And Elimination Reactions Practice Problems
Mar 20, 2025
-
What Is Gas To Solid Called
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Rf Value In Thin Layer Chromatography . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
