Solids Have A Definite Shape Because
Muz Play
Mar 26, 2025 · 6 min read
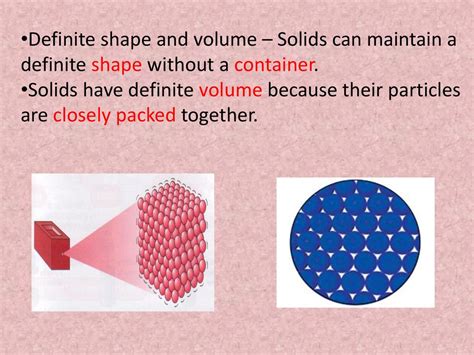
Table of Contents
Solids Have a Definite Shape Because… A Deep Dive into Intermolecular Forces
Solids possess a definite shape and volume, unlike liquids and gases. This fundamental property isn't a coincidence; it's a direct consequence of the strong intermolecular forces holding their constituent particles together. This article will explore the various factors contributing to this characteristic rigidity of solids, delving into the microscopic world to understand the macroscopic behavior we observe.
The Role of Intermolecular Forces
The key to understanding why solids have a definite shape lies in the strength and nature of the intermolecular forces between their atoms, ions, or molecules. These forces dictate how closely packed the particles are and how strongly they are bound to each other. Unlike gases where particles are far apart and move freely, and liquids where particles are close but can slide past one another, solids exhibit strong attractive forces that restrict movement.
Types of Intermolecular Forces: A Hierarchy of Attraction
Several types of intermolecular forces contribute to the overall strength of bonding in solids. Understanding their relative strengths is crucial to grasping the rigidity of different solid types.
-
Ionic Bonds: These are strong electrostatic attractions between oppositely charged ions, forming crystalline structures like those found in table salt (NaCl). The strong Coulombic forces hold ions in fixed positions within the crystal lattice, resulting in a high degree of rigidity and a definite shape.
-
Covalent Bonds: These involve the sharing of electrons between atoms, resulting in strong bonds. In solids, these bonds create extensive networks or large molecules, leading to a highly ordered and rigid structure. Diamond, a network covalent solid, is an excellent example of this strength, showcasing its hardness and definite shape.
-
Metallic Bonds: In metallic solids, the valence electrons are delocalized, forming a "sea" of electrons surrounding positively charged metal ions. This "electron sea" model explains the high electrical and thermal conductivity of metals, but also contributes to their structural rigidity. The strong electrostatic attraction between the positive ions and the electron cloud holds the metal atoms together in a defined structure.
-
Van der Waals Forces: These are weaker forces compared to ionic, covalent, and metallic bonds. They encompass several types of interactions, including London Dispersion Forces (LDFs), dipole-dipole interactions, and hydrogen bonds. While individually weaker, the cumulative effect of these forces in many molecules can still contribute significantly to the overall structure and rigidity of a solid, particularly in molecular solids.
-
London Dispersion Forces (LDFs): These are present in all molecules and arise from temporary fluctuations in electron distribution. While individually weak, they become significant in larger molecules with many electrons.
-
Dipole-Dipole Interactions: These occur in polar molecules where there's a permanent separation of charge. The positive end of one molecule attracts the negative end of another, contributing to the overall attraction and structure.
-
Hydrogen Bonds: A special type of dipole-dipole interaction involving hydrogen bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine). Hydrogen bonds are relatively strong and play a crucial role in determining the properties of many biological molecules and some solids.
-
The Influence of Crystal Structure
The arrangement of particles in a solid, its crystal structure, also significantly influences its shape. Solids are not just a random jumble of particles; they are highly ordered, often forming repeating patterns called crystal lattices. These lattices are determined by the nature of the intermolecular forces and the size and shape of the constituent particles.
Several common crystal systems exist, including cubic, tetragonal, orthorhombic, monoclinic, triclinic, hexagonal, and rhombohedral, each with its unique arrangement of particles. The specific arrangement within these systems influences the macroscopic properties, including the solid's overall shape, hardness, cleavage properties (how it breaks), and other physical characteristics.
The Role of Temperature and Pressure
While intermolecular forces are the primary determinants of a solid's shape, temperature and pressure also play significant roles.
Temperature's Effect
Increasing temperature increases the kinetic energy of the particles within a solid. This increased energy can overcome some of the intermolecular forces, leading to vibrations and, at a high enough temperature, to a phase transition to a liquid (melting). The shape of a solid can subtly change with temperature due to thermal expansion, although this effect is usually small unless the temperature change is substantial.
Pressure's Effect
Applying external pressure can also affect a solid's shape. High pressure can force particles closer together, increasing the strength of intermolecular forces and potentially altering the crystal structure. This can lead to changes in density and even phase transitions. However, the change in shape due to pressure is generally less significant than the effect of intermolecular forces themselves.
Different Types of Solids and Their Definite Shapes
The strength and type of bonding significantly influence the properties and shape of solids.
Crystalline Solids
Crystalline solids are characterized by a highly ordered arrangement of particles, forming a repeating crystal lattice. This ordered structure gives rise to their definite shapes, often exhibiting flat surfaces and well-defined angles. Examples include table salt (NaCl), diamonds, and quartz. The regularity of the crystal lattice directly contributes to the overall definite shape.
Amorphous Solids
Amorphous solids, also known as non-crystalline solids, lack the long-range order found in crystalline solids. Their particles are arranged randomly, resulting in an irregular structure and an absence of a definite, sharp melting point. Examples include glass, rubber, and plastics. While they maintain a definite shape at room temperature, their structure is less rigid than crystalline solids, and their shapes can be more easily altered.
Exceptions and Considerations
While most solids exhibit a definite shape, some exceptions exist:
-
Solids under extreme conditions: Under extreme pressure or temperature, the behavior of solids can deviate from typical expectations. The strong forces holding the structure can be overcome, leading to structural changes and a loss of the defined shape.
-
Flexible solids: Some solids, such as polymers and certain metals, possess some degree of flexibility and can deform under stress without breaking. While their shape can be changed, they generally return to a preferred shape when the stress is removed.
Conclusion: A Strong Foundation of Shape
In conclusion, the definite shape of a solid is a direct consequence of the strong intermolecular forces holding its constituent particles in a fixed, ordered arrangement. The type and strength of these forces, along with the crystal structure, temperature, and pressure, all contribute to this fundamental property. From the strong ionic bonds in salts to the intricate network of covalent bonds in diamond and the delocalized electrons in metals, the microscopic interactions underpin the macroscopic observation of a solid's definite shape. Understanding this relationship allows us to predict and explain the behavior of various solid materials, leading to advancements in material science and engineering.
Latest Posts
Latest Posts
-
Us History Reconstruction To The Present
Mar 29, 2025
-
Label The Structures Of The Upper Respiratory System
Mar 29, 2025
-
Assigning R And S Configuration Practice
Mar 29, 2025
-
Does Higher Pka Mean Stronger Acid
Mar 29, 2025
-
In A Chemical Reaction What Are The Reactants And Products
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Solids Have A Definite Shape Because . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
