Solids To Gases Row Or Column
Muz Play
Mar 22, 2025 · 5 min read
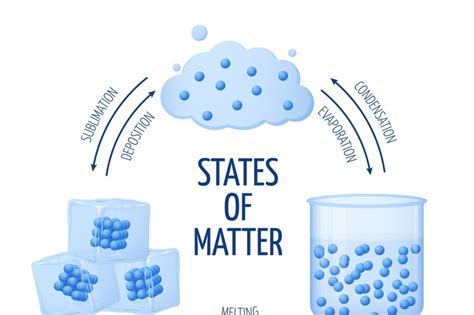
Table of Contents
Solids to Gases: Navigating the Rows and Columns of the Periodic Table
The transition of matter from a solid state directly to a gaseous state, bypassing the liquid phase, is a fascinating phenomenon known as sublimation. Understanding this process requires a nuanced understanding of intermolecular forces, energy, and the organization of elements within the periodic table. While there isn't a strict "row" or "column" prediction for sublimation, analyzing the periodic table reveals trends and patterns that significantly influence a substance's propensity to sublimate. This article delves deep into the relationship between the periodic table and sublimation, exploring the factors influencing this phase transition.
Understanding Sublimation: A Molecular Perspective
Sublimation occurs when the molecules within a solid gain enough kinetic energy to overcome the attractive forces holding them together in a fixed lattice structure. This typically happens at temperatures and pressures below the substance's triple point – the specific temperature and pressure at which the solid, liquid, and gaseous phases coexist in equilibrium. The energy required to break these intermolecular forces is crucial. Stronger intermolecular forces, like those found in ionic compounds or substances with extensive hydrogen bonding, require significantly more energy to overcome, making sublimation less likely.
The Role of Intermolecular Forces
The strength of intermolecular forces is a dominant factor determining whether a solid will sublimate. Let's examine some key types:
-
Van der Waals forces: These weak forces are present in all molecules but are particularly significant in nonpolar molecules. Substances with only weak Van der Waals forces are more likely to sublimate. These forces increase with molecular size and complexity, so larger molecules in a row might exhibit a higher sublimation tendency, although other factors like pressure and temperature also play significant roles.
-
Dipole-dipole interactions: These forces exist between polar molecules and are stronger than Van der Waals forces. Sublimation is less common for substances with strong dipole-dipole interactions.
-
Hydrogen bonding: This exceptionally strong type of dipole-dipole interaction occurs when hydrogen is bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine). Substances with extensive hydrogen bonding, such as ice, typically require substantial energy to transition directly to a gas, making sublimation less probable at standard conditions.
Periodic Table Trends and Sublimation
While no single row or column perfectly predicts sublimation, analyzing the periodic table reveals trends that can be insightful:
-
Across a Period (Row): As we move across a period from left to right, the atomic size generally decreases, and the electronegativity increases. This leads to stronger intermolecular forces (especially in non-metals), reducing the likelihood of sublimation. For example, comparing sodium (Na) and chlorine (Cl) in the third period, sodium, a metal with relatively weak metallic bonding, is less prone to sublimation at standard conditions than chlorine, where stronger dipole-dipole interactions exist in its molecular form (Cl₂).
-
Down a Group (Column): As we move down a group, the atomic size increases, leading to increased polarizability and potentially stronger Van der Waals forces. However, the increase in atomic mass often overshadows this, potentially slowing the sublimation rate. The heavier elements in a group may not necessarily sublimate more readily due to other factors that could outweigh the increase in Van der Waals forces. This highlights the complex interplay of factors that influence sublimation.
Specific Examples and Exceptions
Let's examine some specific elements and compounds to illustrate the complexities:
-
Iodine (I₂): Iodine is a well-known example of a substance that readily sublimates at standard conditions. Its relatively weak intermolecular forces (Van der Waals) allow it to transition directly from solid to gas easily. This behavior is common among halogens.
-
Carbon Dioxide (CO₂): Dry ice (solid CO₂) sublimates readily at standard atmospheric pressure. This is due to the relatively weak intermolecular forces present in CO₂ molecules, primarily Van der Waals forces. The linear shape and non-polar nature of CO₂ minimize intermolecular interactions.
-
Water (H₂O): Water, while possessing strong hydrogen bonding, can sublimate under certain conditions. This is particularly noticeable in extremely cold and dry environments, like high altitudes. However, it's much less likely to sublimate than iodine or carbon dioxide.
-
Metals: Metals generally exhibit high melting and boiling points due to strong metallic bonding. Sublimation is rare for metals under normal conditions. However, under specific conditions, such as in a vacuum, some metals can exhibit sublimation at high temperatures.
Factors Beyond the Periodic Table
The periodic table provides a framework for understanding trends, but other factors significantly influence sublimation:
-
Temperature: Higher temperatures increase the kinetic energy of molecules, making sublimation more likely.
-
Pressure: Lower pressures reduce the external pressure on the solid, making it easier for molecules to escape into the gaseous phase. This is why sublimation is more common at lower pressures.
-
Surface area: A larger surface area exposes more molecules to the surrounding environment, increasing the rate of sublimation.
-
Impurities: The presence of impurities can alter the intermolecular forces within a solid, potentially affecting the sublimation process.
Applications of Sublimation
Sublimation has various applications in various fields:
-
Purification: Sublimation can be used to purify substances, separating volatile components from less volatile impurities.
-
Freeze-drying: This technique utilizes sublimation to remove water from frozen materials, preserving the quality and integrity of the product (e.g., freeze-dried coffee or fruits).
-
Microelectronics: Sublimation is used in the deposition of thin films in microelectronics manufacturing.
-
Art and Photography: Sublimation is used in creating unique prints on fabrics or other materials.
Conclusion
While the periodic table offers valuable insights into the trends affecting sublimation, it's not a definitive predictor. The interplay of intermolecular forces, temperature, pressure, and surface area all influence a substance's propensity to sublimate. Understanding these factors is crucial for predicting and controlling this fascinating phase transition and its various applications across numerous scientific and industrial fields. Further research, beyond simply examining rows and columns, is essential for a complete understanding of the complex phenomena governing sublimation. This involves considering the intricate molecular structures and their corresponding intermolecular interactions, often employing computational modeling and advanced experimental techniques. Analyzing a substance's phase diagram provides critical insight into its behavior under varying conditions of temperature and pressure, revealing the conditions most conducive to sublimation. The study of sublimation continues to be an active area of research, constantly revealing new insights into the behavior of matter.
Latest Posts
Latest Posts
-
Formula For Confidence Interval For Population Proportion
Mar 23, 2025
-
Group 11 On The Periodic Table
Mar 23, 2025
-
Exothermic Or Endothermic Chemical Formula Calculator
Mar 23, 2025
-
How To Solve 3 Equations With 3 Unknowns
Mar 23, 2025
-
Two Recent Periods Of Large Scale Bureaucratic Expansion Were
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Solids To Gases Row Or Column . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
