Strong And Weak Acids And Bases List
Muz Play
Mar 17, 2025 · 6 min read
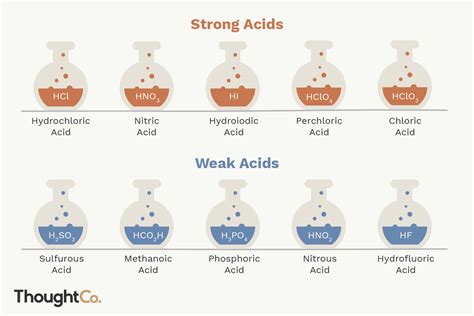
Table of Contents
Strong and Weak Acids and Bases: A Comprehensive List and Explanation
Understanding the strength of acids and bases is fundamental to chemistry. This comprehensive guide will delve into the differences between strong and weak acids and bases, providing detailed explanations and extensive lists of common examples. We'll explore the concepts of dissociation, pH, and pKa, illustrating how these factors determine acid and base strength. By the end, you'll have a solid grasp of this crucial chemical concept.
What Makes an Acid or Base Strong or Weak?
The strength of an acid or base is determined by its degree of dissociation in water. This refers to the extent to which the acid or base breaks apart into its constituent ions.
- Strong acids and bases completely dissociate in water. This means that virtually every molecule of the acid or base separates into ions.
- Weak acids and bases only partially dissociate in water. A significant portion of the molecules remain undissociated, existing in equilibrium with their ions.
This difference in dissociation directly impacts the concentration of H⁺ (hydronium) ions (for acids) or OH⁻ (hydroxide) ions (for bases) in solution, leading to variations in pH and impacting their reactivity.
Strong Acids: Complete Dissociation and High Acidity
Strong acids are characterized by their complete dissociation in aqueous solutions. This results in a high concentration of H⁺ ions, leading to a low pH (typically below 3). The following is a list of common strong acids:
List of Common Strong Acids:
- Hydrochloric acid (HCl): Found in gastric acid and used extensively in industrial processes.
- Hydrobromic acid (HBr): A highly corrosive acid used in various chemical syntheses.
- Hydroiodic acid (HI): Another highly corrosive acid with applications in organic chemistry.
- Nitric acid (HNO₃): Used in the production of fertilizers and explosives.
- Sulfuric acid (H₂SO₄): A crucial industrial chemical used in the production of fertilizers, batteries, and many other products. Note that it dissociates in two steps, but the first step is essentially complete, making it a strong acid.
- Perchloric acid (HClO₄): A very strong and powerful oxidizing agent.
Important Note: The strength of an acid is not directly related to its concentration. A dilute solution of a strong acid is still a strong acid, even though its H⁺ ion concentration is lower than that of a concentrated solution of the same acid. The key is the degree of dissociation, not the absolute amount of acid present.
Weak Acids: Partial Dissociation and Moderate Acidity
Weak acids only partially dissociate in water. This means that an equilibrium exists between the undissociated acid molecules and their constituent ions. The extent of dissociation is quantified by the acid dissociation constant (Ka). A smaller Ka value indicates a weaker acid. The negative logarithm of Ka, pKa, is often used instead as a smaller pKa value indicates a stronger acid.
List of Common Weak Acids:
- Acetic acid (CH₃COOH): Found in vinegar, a common household acid.
- Formic acid (HCOOH): The simplest carboxylic acid, found in ant stings.
- Citric acid (C₆H₈O₇): Found in citrus fruits.
- Benzoic acid (C₇H₆O₂): Used as a preservative and in the synthesis of other chemicals.
- Carbonic acid (H₂CO₃): Present in carbonated drinks and plays a vital role in blood buffering.
- Phosphoric acid (H₃PO₄): Used in fertilizers and food additives. It's a triprotic acid, meaning it can donate three protons, but each dissociation step has a different Ka value.
- Hydrofluoric acid (HF): Surprisingly, despite fluorine being highly electronegative, HF is a relatively weak acid. This is due to the strong hydrogen bonding in aqueous solutions.
Strong Bases: Complete Dissociation and High Basicity
Similar to strong acids, strong bases completely dissociate in water, producing a high concentration of hydroxide ions (OH⁻). This leads to a high pH (typically above 11).
List of Common Strong Bases:
- Sodium hydroxide (NaOH): Commonly known as lye or caustic soda, used in many industrial and household applications.
- Potassium hydroxide (KOH): Another strong base, similar in properties to NaOH.
- Lithium hydroxide (LiOH): Used in some batteries and in the absorption of carbon dioxide.
- Calcium hydroxide (Ca(OH)₂): Also known as slaked lime, used in construction and water treatment.
- Barium hydroxide (Ba(OH)₂): A strong base used in certain chemical reactions.
- Strontium hydroxide (Sr(OH)₂): Similar to barium hydroxide in its properties.
Weak Bases: Partial Dissociation and Moderate Basicity
Weak bases only partially dissociate in water, forming a relatively low concentration of hydroxide ions. Their strength is also described by a base dissociation constant (Kb) or its negative logarithm, pKb. A smaller Kb or pKb value indicates a stronger base.
List of Common Weak Bases:
- Ammonia (NH₃): A common household cleaner and an important industrial chemical.
- Methylamine (CH₃NH₂): A simple organic base used in various chemical syntheses.
- Aniline (C₆H₅NH₂): An aromatic amine used in the dye industry.
- Pyridine (C₅H₅N): A heterocyclic aromatic base with applications in organic chemistry.
- Many amines (R-NH₂): Organic compounds containing nitrogen and hydrogen form a large class of weak bases. The strength varies depending on the nature of the R group.
- Phosphate ions (PO₄³⁻, HPO₄²⁻, H₂PO₄⁻): These are conjugate bases of phosphoric acid and exhibit weak basicity.
Understanding pH and pKa/pKb
pH is a measure of the acidity or basicity of a solution, defined as the negative logarithm of the hydrogen ion concentration ([H⁺]). A lower pH indicates a higher acidity, while a higher pH indicates a higher basicity. A pH of 7 is neutral.
pKa (for acids) and pKb (for bases) are related to the acid and base dissociation constants and provide a measure of the relative strength of the acid or base. A lower pKa value indicates a stronger acid, and a lower pKb value indicates a stronger base. The relationship between pKa and pKb for a conjugate acid-base pair is given by: pKa + pKb = 14 at 25°C.
Factors Affecting Acid and Base Strength
Several factors influence the strength of an acid or base:
-
Electronegativity: In general, the higher the electronegativity of the atom bonded to hydrogen in an acid, the stronger the acid. This is because the more electronegative atom pulls the electron density away from the hydrogen, making it easier to release as a proton.
-
Bond strength: Weaker bonds between the acidic hydrogen and the rest of the molecule lead to stronger acids, as the bond is more easily broken.
-
Size of the anion: Larger anions are more stable, leading to stronger acids. This is because the negative charge is more spread out over a larger area.
-
Resonance: The presence of resonance structures in the conjugate base stabilizes the anion, making the acid stronger.
-
Inductive effects: Electron-withdrawing groups increase the acidity, while electron-donating groups decrease it.
Practical Applications of Strong and Weak Acids and Bases
The distinction between strong and weak acids and bases is crucial in numerous applications:
-
Industrial processes: Strong acids and bases are used in various industrial processes, such as the production of fertilizers, plastics, and detergents.
-
Pharmaceuticals: Understanding acid-base chemistry is essential in the development and formulation of pharmaceuticals.
-
Environmental science: Acid rain and water pollution are directly related to the presence of strong and weak acids and bases.
-
Biological systems: Many biological processes rely on the properties of weak acids and bases, such as buffering systems in blood.
-
Analytical chemistry: Titrations and other analytical techniques depend on the principles of acid-base chemistry.
This comprehensive guide has explored the crucial differences between strong and weak acids and bases, providing extensive lists and explanations to enhance your understanding of this fundamental chemical concept. Remember that understanding the degree of dissociation, pH, pKa, and pKb, alongside other influencing factors, is vital for effective application in various scientific fields.
Latest Posts
Latest Posts
-
Protons Neutrons And Electrons For Helium
Mar 17, 2025
-
Magnetic Field In A Bar Magnet
Mar 17, 2025
-
Competes With Substrate For Binding To An Active Site
Mar 17, 2025
-
Which Compound Is Soluble In Water
Mar 17, 2025
-
Life Cycle Of Seedless Vascular Plants
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Strong And Weak Acids And Bases List . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
