The Energy Needed To Start A Chemical Reaction Is Called
Muz Play
Mar 31, 2025 · 6 min read
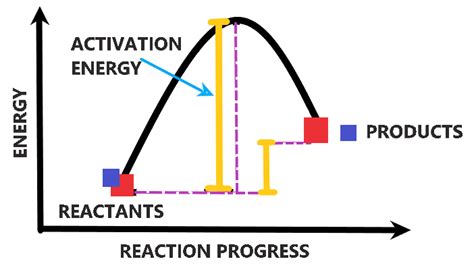
Table of Contents
The Energy Needed to Start a Chemical Reaction: Activation Energy Explained
The energy needed to initiate a chemical reaction is called activation energy. This fundamental concept in chemistry governs the rate at which reactions proceed, impacting everything from biological processes within our bodies to industrial manufacturing processes. Understanding activation energy is key to comprehending reaction kinetics and controlling chemical transformations. This comprehensive guide delves into the intricacies of activation energy, exploring its definition, significance, factors influencing it, and real-world applications.
What is Activation Energy?
Activation energy (Ea) is the minimum amount of energy required to start a chemical reaction. Think of it as the energy barrier that reactant molecules must overcome to transform into products. It's not the overall energy change of the reaction (ΔH), which represents the difference in energy between reactants and products, but rather the energy needed to reach the transition state. The transition state is a high-energy, unstable intermediate configuration of atoms that exists momentarily before the reactants rearrange to form products.
Imagine pushing a boulder uphill. The energy you expend to get the boulder to the top of the hill represents the activation energy. Once it's at the top, it can roll down the other side, releasing energy. This downhill roll represents the overall energy change of the reaction (exothermic reaction, where energy is released). If the boulder needs a significant push to reach the top (high activation energy), it will roll down slowly. If the push is smaller (low activation energy), it will roll down quickly.
Activation Energy and Reaction Rates
Activation energy is inversely proportional to the reaction rate. A lower activation energy leads to a faster reaction rate, as more reactant molecules possess sufficient energy to overcome the energy barrier. Conversely, a higher activation energy leads to a slower reaction rate. This relationship is elegantly described by the Arrhenius equation:
k = Ae^(-Ea/RT)
Where:
- k is the rate constant (a measure of reaction speed)
- A is the pre-exponential factor (related to the frequency of collisions between reactant molecules)
- Ea is the activation energy
- R is the ideal gas constant
- T is the absolute temperature
This equation shows that increasing the temperature (T) exponentially increases the reaction rate (k), as more molecules gain enough energy to surpass the activation energy barrier.
Factors Affecting Activation Energy
Several factors influence the activation energy of a chemical reaction:
1. Nature of Reactants:
The inherent properties of the reacting molecules play a crucial role. Strong bonds require more energy to break than weak bonds, consequently leading to a higher activation energy. The size and complexity of molecules also influence the activation energy. Larger, more complex molecules often have higher activation energies due to the increased number of bonds that need to be broken and reformed.
2. Reaction Mechanism:
The specific pathway a reaction takes significantly impacts the activation energy. Reactions often proceed through a series of intermediate steps, each with its own activation energy. The overall activation energy is determined by the highest energy barrier along the reaction pathway (the rate-determining step). A reaction mechanism with a rate-determining step involving a high-energy transition state will exhibit a higher activation energy.
3. Presence of a Catalyst:
Catalysts are substances that accelerate reaction rates without being consumed in the process. They achieve this by providing an alternative reaction pathway with a lower activation energy. Catalysts typically do this by forming intermediate complexes with the reactants, which lower the energy barrier required for the reaction to proceed. Enzymes are biological catalysts that play a vital role in regulating biochemical reactions within living organisms.
4. Temperature:
As mentioned earlier, temperature directly affects the reaction rate. At higher temperatures, a larger fraction of reactant molecules possess sufficient kinetic energy to overcome the activation energy barrier, resulting in a faster reaction rate. This is why many reactions proceed much faster at elevated temperatures.
5. Concentration of Reactants:
The concentration of reactants also influences the reaction rate. Higher reactant concentrations lead to more frequent collisions between reactant molecules, increasing the probability that molecules will possess sufficient energy to overcome the activation energy barrier, thereby increasing the reaction rate.
Activation Energy and Reaction Types
The magnitude of activation energy is crucial in classifying reaction types:
1. Exothermic Reactions:
In exothermic reactions, the energy of the products is lower than the energy of the reactants. Even though energy is released overall, an initial input of energy (activation energy) is still required to initiate the reaction. The activation energy is reflected in the energy barrier between reactants and products.
2. Endothermic Reactions:
In endothermic reactions, the energy of the products is higher than the energy of the reactants. The activation energy is greater in endothermic reactions because additional energy must be supplied to reach the higher energy state of the products.
Real-World Applications of Activation Energy
Understanding activation energy has wide-ranging applications across various scientific fields:
1. Industrial Chemistry:
In industrial processes, controlling reaction rates is crucial for efficient and economical production. Manipulating activation energy through temperature control, catalyst use, or reactant concentration optimization is essential for maximizing product yield and minimizing waste.
2. Biochemistry and Biology:
Activation energy is paramount in understanding biochemical reactions within living organisms. Enzymes, acting as biological catalysts, drastically lower the activation energy of metabolic reactions, allowing them to occur at physiologically relevant temperatures and rates. Understanding enzyme kinetics, including their activation energies, is crucial for comprehending biological processes, developing pharmaceuticals, and diagnosing diseases.
3. Environmental Science:
Activation energy plays a vital role in understanding chemical transformations in the environment. For instance, the decomposition of pollutants often requires a certain activation energy. This knowledge is essential for designing effective strategies for environmental remediation.
4. Materials Science:
The synthesis and properties of materials are often determined by the activation energy of the chemical reactions involved in their formation. By controlling activation energy, scientists can tailor material properties to specific applications, such as developing new catalysts, polymers, or semiconductors.
Measuring Activation Energy
Activation energy can be determined experimentally using various methods, primarily by measuring the reaction rate at different temperatures. The Arrhenius equation, previously mentioned, forms the basis for these calculations. By plotting the natural logarithm of the rate constant (ln k) against the reciprocal of the absolute temperature (1/T), a linear relationship is obtained, with the slope equal to -Ea/R. From this slope, the activation energy (Ea) can be calculated.
Conclusion: Activation Energy – A Cornerstone of Chemistry
Activation energy is a fundamental concept that underpins our understanding of chemical reactions and their rates. Its influence spans diverse fields, from industrial processes to biological systems, making its study crucial for advancing scientific knowledge and technological innovation. By controlling activation energy, scientists and engineers can manipulate reaction rates, optimize processes, and develop new materials and technologies. The continuous exploration of activation energy and its influencing factors promises further breakthroughs in various scientific disciplines. A deeper understanding of this concept remains vital in unlocking the secrets of chemical transformations and harnessing their potential for the benefit of society.
Latest Posts
Latest Posts
-
What Is The Chromosomal Basis Of Inheritance
Apr 02, 2025
-
Two Different Ionic Compounds Each Contain
Apr 02, 2025
-
Part Ii Equilibria Involving Sparingly Soluble Salts
Apr 02, 2025
-
Adding Strong Acid To A Buffer
Apr 02, 2025
-
The Most Reactive Group In The Periodic Table
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about The Energy Needed To Start A Chemical Reaction Is Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
