The Graph Shows The Oxygen-binding Curves For Myoglobin And Hemoglobin
Muz Play
Mar 23, 2025 · 6 min read
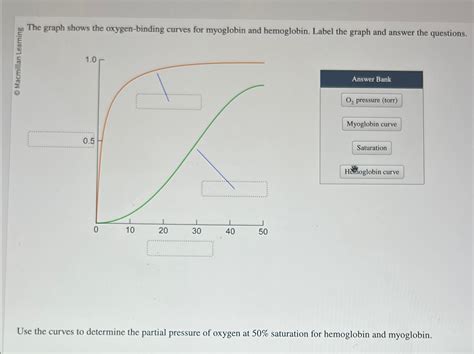
Table of Contents
The Graph Shows the Oxygen-Binding Curves for Myoglobin and Hemoglobin: A Deep Dive into Muscle Oxygenation and Blood Transport
The image of oxygen-binding curves for myoglobin and hemoglobin is a cornerstone of understanding oxygen transport in the body. These curves, visually distinct, reveal the fundamental differences in the oxygen-binding affinities of these two crucial proteins and their crucial roles in oxygen delivery to tissues. This article will delve into a detailed explanation of these curves, exploring their shapes, the underlying mechanisms, physiological significance, and the impact of various factors influencing oxygen binding.
Understanding the Oxygen-Binding Curves
The oxygen-binding curve graphically represents the relationship between the partial pressure of oxygen (PO2) and the percentage of oxygen saturation (% saturation) of either myoglobin or hemoglobin. The x-axis represents the PO2 (in mmHg), and the y-axis represents the percentage saturation of the protein with oxygen.
Myoglobin's Oxygen-Binding Curve: This curve is characterized by its hyperbolic shape. It signifies a high affinity of myoglobin for oxygen. Even at low PO2 values (such as those found in resting muscle tissue), myoglobin remains significantly saturated with oxygen. This high affinity ensures that myoglobin acts as an effective oxygen storage protein within muscle cells. Once oxygen is bound, it is held tightly, releasing it only when the PO2 drops drastically.
Hemoglobin's Oxygen-Binding Curve: This curve demonstrates a sigmoidal shape. This sigmoidal shape, unlike the hyperbolic curve of myoglobin, reflects hemoglobin's cooperative binding of oxygen. The curve's initial slope is relatively shallow, indicating a lower affinity for oxygen at low PO2 values. However, as PO2 increases, the slope steepens, representing a rapid increase in oxygen saturation. This sigmoidal shape is critical to hemoglobin's function as an efficient oxygen transporter.
The Cooperative Binding of Oxygen to Hemoglobin: A Closer Look
Hemoglobin's sigmoidal curve is a direct consequence of its cooperative binding of oxygen. Hemoglobin is a tetrameric protein composed of four subunits: two alpha and two beta subunits. Each subunit contains a heme group, which binds one oxygen molecule. The binding of the first oxygen molecule to one subunit induces a conformational change in the entire hemoglobin molecule, increasing the affinity for oxygen in the remaining subunits. This positive cooperativity makes oxygen binding more efficient at higher PO2 values, such as in the lungs. Conversely, the release of oxygen from one subunit facilitates the release of oxygen from other subunits, enhancing oxygen unloading in tissues with low PO2.
The Importance of Allosteric Regulation
The cooperative binding behavior of hemoglobin is a prime example of allosteric regulation. Allosteric regulation refers to the change in the protein's activity or binding affinity due to the binding of a molecule at a site other than the active site. In the case of hemoglobin, the binding of oxygen to one subunit alters the conformation of the entire molecule, thus affecting the oxygen-binding affinity of other subunits. This allosteric interaction is key to hemoglobin's ability to efficiently load oxygen in the lungs and unload it in the tissues.
The Physiological Significance of the Differences
The distinct oxygen-binding curves of myoglobin and hemoglobin are perfectly adapted to their specific roles in oxygen transport and storage.
Myoglobin's Role as an Oxygen Storage Protein: Myoglobin's high oxygen affinity ensures that it readily binds oxygen when PO2 is high, such as in capillaries supplying the muscle. This stored oxygen serves as a reserve, readily available to support muscle activity during periods of increased oxygen demand (e.g., exercise). The tight binding prevents premature release of oxygen, ensuring oxygen is available when needed.
Hemoglobin's Role as an Oxygen Transporter: Hemoglobin's cooperative binding and sigmoidal curve are crucial for efficient oxygen transport. In the lungs, where PO2 is high (around 100 mmHg), hemoglobin efficiently loads with oxygen. As blood travels to the tissues, where PO2 is lower (around 40 mmHg), the cooperative binding ensures that a significant portion of the bound oxygen is released to support cellular respiration. This efficient loading and unloading are paramount for maintaining sufficient oxygen supply to various tissues throughout the body.
Factors Affecting Hemoglobin's Oxygen-Binding Affinity
Several factors influence hemoglobin's oxygen-binding affinity and, consequently, the shape and position of its oxygen-binding curve. These factors are crucial for regulating oxygen delivery in response to physiological demands.
pH (The Bohr Effect):
The Bohr effect describes the impact of pH on hemoglobin's oxygen-binding affinity. A decrease in pH (increased acidity), such as during strenuous exercise, reduces hemoglobin's affinity for oxygen, facilitating oxygen release to the tissues. This is because increased acidity promotes the formation of certain hemoglobin conformations that have a lower oxygen affinity.
Temperature:
Increased temperature also reduces hemoglobin's affinity for oxygen, promoting oxygen release. This effect is particularly significant during intense physical activity when muscle temperature rises.
2,3-Bisphosphoglycerate (2,3-BPG):
2,3-BPG is a molecule present in red blood cells. It binds to hemoglobin, reducing its oxygen affinity and facilitating oxygen release in the tissues. The levels of 2,3-BPG increase in response to various stimuli, including high altitude, where oxygen availability is reduced. This adaptation ensures that a greater proportion of oxygen is unloaded to the tissues despite the lower PO2.
Carbon Dioxide (CO2):
Carbon dioxide also influences hemoglobin's oxygen-binding affinity, acting as an allosteric regulator. Increased CO2 levels reduce hemoglobin's oxygen affinity, promoting oxygen unloading in tissues. This effect is synergistic with the Bohr effect, contributing to more efficient oxygen delivery during periods of high metabolic activity.
Clinical Significance and Applications
Understanding the oxygen-binding curves of myoglobin and hemoglobin has crucial clinical implications. Conditions affecting either protein can significantly impact oxygen transport and tissue oxygenation.
Myoglobinuria: Damage to muscle tissue, such as during a crush injury or strenuous exercise, can lead to the release of myoglobin into the bloodstream. This condition, known as myoglobinuria, can cause kidney damage due to the toxic effects of myoglobin on the kidneys.
Anemias: Various anemias are characterized by a reduced capacity of the blood to carry oxygen. These can arise from deficiencies in hemoglobin production, abnormal hemoglobin structure (e.g., sickle cell anemia), or reduced red blood cell count. Understanding the oxygen-binding curve helps in diagnosing and managing these conditions.
Carbon Monoxide Poisoning: Carbon monoxide (CO) binds to hemoglobin with much greater affinity than oxygen, effectively preventing oxygen from binding. This results in significantly reduced oxygen-carrying capacity, leading to hypoxia (oxygen deficiency) and potentially fatal consequences.
Conclusion
The oxygen-binding curves for myoglobin and hemoglobin are visually distinct yet functionally complementary, illustrating the elegant design of oxygen transport and storage in the body. The hyperbolic curve of myoglobin reflects its role as an oxygen storage protein, while the sigmoidal curve of hemoglobin highlights its cooperative binding and efficient oxygen transport capabilities. Understanding these curves, the underlying mechanisms, and the influence of various factors provides valuable insight into the complex physiology of oxygen delivery and its clinical relevance. Further research continues to expand our understanding of these crucial proteins and their roles in health and disease. By studying these curves, we can better appreciate the intricate interplay of biological factors that maintain adequate oxygen levels for cellular respiration, highlighting the vital importance of both myoglobin and hemoglobin for sustaining life.
Latest Posts
Latest Posts
-
Common Particles With Charge Of 2
Mar 25, 2025
-
What Does The Top Command Do In Linux
Mar 25, 2025
-
Picture Of A Cell In Interphase
Mar 25, 2025
-
In Biology How Is A Weed Defined
Mar 25, 2025
-
What Is The Ph Of The Neutral Solution
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about The Graph Shows The Oxygen-binding Curves For Myoglobin And Hemoglobin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
