The Movement Of Materials From High To Low Concentration
Muz Play
Mar 26, 2025 · 6 min read
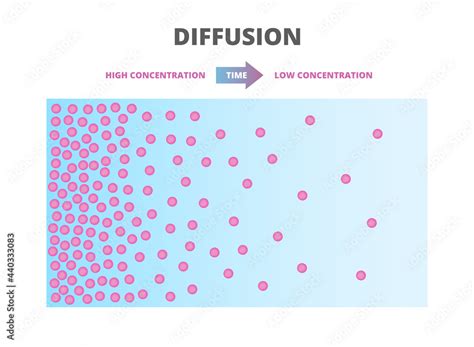
Table of Contents
The Movement of Materials from High to Low Concentration: A Deep Dive into Diffusion and Osmosis
The movement of materials from regions of high concentration to regions of low concentration is a fundamental principle governing numerous processes in biology, chemistry, and physics. This principle, driven by the second law of thermodynamics, dictates the spontaneous tendency of systems to increase entropy, or disorder. This article will delve into the intricacies of this movement, exploring the mechanisms of diffusion, osmosis, and facilitated diffusion, alongside their significance in various contexts.
Understanding Concentration Gradients
Before we delve into the specific mechanisms, let's establish a clear understanding of concentration gradients. A concentration gradient exists whenever there's an unequal distribution of a substance within a space. Imagine a drop of ink placed in a glass of water. Initially, the ink is highly concentrated at the point of introduction. However, over time, the ink molecules spread out, moving from the area of high concentration to the area of low concentration until equilibrium is reached, resulting in a uniform distribution of ink throughout the water. This movement is driven by the inherent kinetic energy of the molecules and the goal of maximizing entropy.
Diffusion: The Passive Movement of Molecules
Diffusion is the passive movement of molecules or particles from a region of higher concentration to a region of lower concentration. This process requires no energy input and is driven solely by the random motion of particles. The rate of diffusion is influenced by several factors:
Factors Affecting Diffusion Rate:
-
Concentration gradient: A steeper gradient (larger difference in concentration) leads to faster diffusion. The greater the difference, the stronger the driving force.
-
Temperature: Higher temperatures increase the kinetic energy of particles, resulting in faster diffusion. Increased molecular movement means faster spreading.
-
Mass of the particles: Larger, heavier molecules diffuse more slowly than smaller, lighter molecules. Inertia plays a significant role here.
-
Surface area: A larger surface area allows for more molecules to cross the boundary simultaneously, increasing the diffusion rate.
-
Distance: Diffusion is slower over longer distances. The molecules have to travel further to reach equilibrium.
-
Medium: The medium through which diffusion occurs impacts the rate. Diffusion is faster in gases than in liquids, and slowest in solids.
Examples of Diffusion in Biological Systems:
-
Gas exchange in the lungs: Oxygen diffuses from the alveoli (air sacs in the lungs) into the bloodstream, while carbon dioxide diffuses from the blood into the alveoli to be exhaled. This process relies on the concentration gradient between the alveoli and the blood.
-
Nutrient absorption in the small intestine: Nutrients from digested food diffuse across the intestinal lining into the bloodstream. The high concentration of nutrients in the intestine drives this absorption.
-
Neurotransmitter diffusion at synapses: Neurotransmitters, chemical messengers in the nervous system, diffuse across the synaptic cleft (the gap between two nerve cells) to transmit signals. The speed of this diffusion is critical for nervous system function.
Osmosis: The Diffusion of Water Across a Selectively Permeable Membrane
Osmosis is a special case of diffusion that involves the movement of water molecules across a selectively permeable membrane. A selectively permeable membrane allows certain molecules to pass through while restricting the passage of others. In osmosis, water moves from a region of high water concentration (low solute concentration) to a region of low water concentration (high solute concentration). This movement aims to equalize the concentration of solutes on both sides of the membrane.
Osmotic Pressure:
The pressure required to prevent the net movement of water across a selectively permeable membrane is called osmotic pressure. The higher the solute concentration, the higher the osmotic pressure.
Types of Osmotic Solutions:
-
Hypotonic solution: A solution with a lower solute concentration compared to the inside of a cell. Water moves into the cell, potentially causing it to swell or burst (lyse).
-
Hypertonic solution: A solution with a higher solute concentration compared to the inside of a cell. Water moves out of the cell, causing it to shrink (crenate).
-
Isotonic solution: A solution with the same solute concentration as the inside of a cell. There is no net movement of water.
Examples of Osmosis in Biological Systems:
-
Water absorption by plant roots: Water moves from the soil (hypotonic) into the roots (hypertonic) by osmosis, providing essential hydration for the plant.
-
Water reabsorption in the kidneys: The kidneys regulate water balance in the body through osmosis, reabsorbing water from the filtrate back into the bloodstream.
-
Maintaining cell turgor pressure in plants: Osmosis helps maintain the turgor pressure within plant cells, providing structural support.
Facilitated Diffusion: Assisted Passive Transport
Facilitated diffusion is another type of passive transport that involves the movement of molecules across a membrane with the help of transport proteins. These proteins act as channels or carriers, facilitating the movement of specific molecules down their concentration gradient. While it's still passive (no energy input is required), it provides a more controlled and efficient way for certain molecules to cross the membrane.
Types of Transport Proteins:
-
Channel proteins: Form hydrophilic pores or channels in the membrane, allowing specific molecules to pass through.
-
Carrier proteins: Bind to specific molecules and undergo conformational changes to transport them across the membrane.
Examples of Facilitated Diffusion:
-
Glucose transport into cells: Glucose, an essential sugar, enters cells through facilitated diffusion using glucose transporter proteins.
-
Ion transport across cell membranes: Ions like sodium, potassium, and calcium are transported across cell membranes via ion channels, playing crucial roles in nerve impulse transmission and muscle contraction.
Active Transport: Moving Against the Gradient
Unlike diffusion and osmosis, which are passive processes, active transport requires energy input (typically ATP) to move molecules against their concentration gradient – from a region of low concentration to a region of high concentration. This process is essential for maintaining concentration gradients crucial for cellular function.
Examples of Active Transport:
-
Sodium-potassium pump: This pump maintains the electrochemical gradient across cell membranes by actively transporting sodium ions out of the cell and potassium ions into the cell.
-
Proton pumps: These pumps move protons (H⁺ ions) across membranes, creating a proton gradient used to drive other processes, such as ATP synthesis.
-
Endocytosis and Exocytosis: These processes involve the bulk transport of materials into (endocytosis) and out of (exocytosis) the cell, requiring energy expenditure.
Conclusion: The Importance of Concentration Gradients
The movement of materials from high to low concentration, encompassing diffusion, osmosis, facilitated diffusion, and active transport, is a fundamental process underpinning life itself. Understanding these mechanisms is essential for comprehending a wide range of biological phenomena, from gas exchange in the lungs to nutrient absorption in the intestines, and even the intricacies of nerve impulse transmission. The intricate interplay between these different transport methods ensures that cells and organisms maintain the necessary internal environment for survival and function. Further research into these processes continues to reveal new insights and complexities, solidifying their importance in the study of biology and related fields. The applications extend beyond biology into various chemical and physical systems, highlighting the universal nature of this fundamental principle. The constant quest for equilibrium drives this movement and creates the dynamic environments essential for life and the natural world.
Latest Posts
Latest Posts
-
How To Determine If A Reaction Is Spontaneous
Mar 29, 2025
-
What Bonds Are The Most Polar
Mar 29, 2025
-
Systems Of Linear Equations And Inequalities
Mar 29, 2025
-
Cuanto Pesa Un Galon De Agua
Mar 29, 2025
-
Example Of The First Law Of Thermodynamics
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about The Movement Of Materials From High To Low Concentration . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
