The Number Of Protons In An Atom Is Called What
Muz Play
Mar 18, 2025 · 7 min read
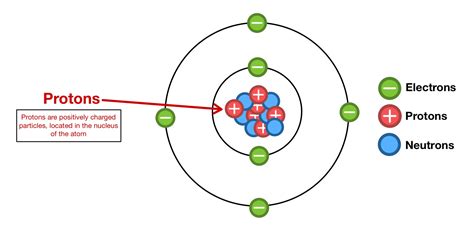
Table of Contents
The Number of Protons in an Atom is Called What? Understanding Atomic Number and its Significance
The number of protons in an atom's nucleus is a fundamental property that defines its identity and chemical behavior. This crucial number is known as the atomic number. Understanding atomic number is key to comprehending the structure of matter, the periodic table, and the behavior of elements in chemical reactions. This article will delve deep into the concept of atomic number, exploring its implications across various branches of science.
What is Atomic Number?
The atomic number, represented by the symbol Z, is the number of protons found in the nucleus of an atom. Protons, along with neutrons, constitute the atom's nucleus, while electrons orbit the nucleus in distinct energy levels. It's crucial to understand that the atomic number uniquely identifies an element. All atoms of a particular element have the same atomic number; conversely, atoms with different atomic numbers represent different elements. For instance, hydrogen (H) has an atomic number of 1, meaning it has one proton in its nucleus. Helium (He), with an atomic number of 2, possesses two protons. This simple yet powerful concept forms the foundation of the periodic table, a systematic organization of elements based on their atomic numbers and recurring chemical properties.
Atomic Number vs. Mass Number
While atomic number refers to the number of protons, mass number (A) represents the total number of protons and neutrons in an atom's nucleus. Neutrons, like protons, are subatomic particles found within the nucleus, but unlike protons, they carry no electrical charge. The mass number provides information about the overall mass of the atom, which is primarily determined by the combined mass of protons and neutrons. Isotopes, which are atoms of the same element with different numbers of neutrons, have the same atomic number but different mass numbers. For example, carbon-12 (¹²C) and carbon-14 (¹⁴C) are isotopes of carbon; both have an atomic number of 6 (six protons), but carbon-12 has six neutrons (mass number 12), while carbon-14 has eight neutrons (mass number 14).
The Significance of Atomic Number
The atomic number's significance extends far beyond simply identifying elements. It plays a crucial role in determining several key atomic properties:
1. Chemical Properties: The Defining Factor</h3>
The atomic number is the primary determinant of an element's chemical properties. This is because the number of protons dictates the number of electrons in a neutral atom (since the number of protons equals the number of electrons to maintain charge neutrality). These electrons are arranged in electron shells and subshells, and the outermost electrons, known as valence electrons, participate in chemical bonding. The configuration of valence electrons determines how an atom will interact with other atoms, forming molecules and compounds. Elements with similar valence electron configurations exhibit similar chemical properties, which is the basis for the organization of the periodic table.
2. Position on the Periodic Table: A Systematic Arrangement</h3>
The periodic table arranges elements in increasing order of their atomic numbers. This arrangement is not arbitrary; it reflects the periodic recurrence of similar chemical properties. Elements in the same column (group) share similar valence electron configurations and therefore exhibit similar chemical behavior. For instance, all elements in Group 1 (alkali metals) have one valence electron, leading to similar reactivity. The periodic table is a powerful tool for predicting the properties of elements based on their atomic numbers and positions within the table.
3. Nuclear Properties: Stability and Radioactivity</h3>
The atomic number also influences an element's nuclear stability. Elements with specific atomic numbers exhibit greater stability than others. Elements with atomic numbers beyond 83 are generally radioactive, meaning their nuclei are unstable and undergo spontaneous decay, emitting particles and energy. This radioactivity has both beneficial and detrimental effects, finding applications in medical imaging and treatment, but also posing environmental hazards. The study of nuclear stability and radioactive decay is crucial in understanding nuclear physics and its applications.
4. Spectroscopic Properties: Unique Fingerprints</h3>
Each element possesses a unique atomic spectrum, which is a characteristic pattern of light emitted or absorbed when electrons transition between energy levels. This spectrum is determined by the element's atomic number and electron configuration. Spectroscopy, the study of these spectra, allows scientists to identify elements based on their unique spectral "fingerprints," even in trace amounts. This technique is widely used in various fields, including astronomy, environmental monitoring, and forensic science.
Exploring the Periodic Table through Atomic Number
The periodic table’s arrangement, fundamentally based on atomic number, allows us to predict and understand the properties of elements. Let's explore some examples:
- Group 1 (Alkali Metals): These elements (Li, Na, K, Rb, Cs, Fr) all have one valence electron, making them highly reactive and readily forming +1 ions. Their reactivity increases as you move down the group.
- Group 17 (Halogens): These elements (F, Cl, Br, I, At) have seven valence electrons, needing only one electron to achieve a stable octet. This makes them highly reactive and prone to forming -1 ions.
- Group 18 (Noble Gases): These elements (He, Ne, Ar, Kr, Xe, Rn) have a full outer electron shell (except for Helium), making them extremely unreactive and chemically inert. Their stability is a direct consequence of their electron configuration, determined by their atomic numbers.
- Transition Metals: These elements occupy the middle section of the periodic table, characterized by variable oxidation states and often forming colorful compounds. Their diverse chemical properties stem from their complex electron configurations and the ability of their d-electrons to participate in bonding.
Applications of Atomic Number Knowledge
The understanding of atomic numbers has led to numerous advancements across diverse scientific fields. Some notable examples include:
- Nuclear Medicine: Radioactive isotopes, many identified by their atomic number and mass number, are used in diagnostic and therapeutic procedures. These isotopes emit radiation that can be detected to image internal organs or target and destroy cancerous cells.
- Materials Science: The properties of materials, such as strength, conductivity, and reactivity, are directly related to the atomic numbers and arrangement of the constituent elements. Understanding atomic numbers helps scientists design new materials with tailored properties.
- Analytical Chemistry: Techniques like atomic absorption spectroscopy and inductively coupled plasma mass spectrometry rely on the unique spectral signatures of elements, determined by their atomic numbers, to quantitatively analyze the composition of samples.
- Astrophysics: The analysis of light from stars and other celestial objects reveals their elemental composition, determined by identifying the atomic numbers associated with spectral lines. This allows astronomers to understand the formation and evolution of stars and galaxies.
Beyond the Basics: Exploring Isotopes and Nuclear Reactions
As mentioned earlier, isotopes are atoms of the same element with differing numbers of neutrons. Although isotopes have the same atomic number (and thus the same chemical properties), they have different mass numbers and may exhibit different nuclear properties. Some isotopes are stable, while others are radioactive, undergoing decay processes governed by their nuclear structure. Understanding isotopic variations is crucial in various applications, including radioactive dating, nuclear medicine, and environmental monitoring.
Nuclear reactions involve changes in the nucleus of an atom, often involving changes in the number of protons and neutrons. These reactions can lead to the formation of new elements, altering their atomic numbers. Understanding nuclear reactions is fundamental to nuclear power generation, nuclear weapons technology, and the study of the origin of elements in the universe.
Conclusion: The Cornerstone of Chemistry and Physics
The atomic number, representing the number of protons in an atom, is a fundamental concept in chemistry and physics. It uniquely identifies each element, dictates its chemical properties, determines its position in the periodic table, and influences its nuclear properties. Understanding atomic number is essential for comprehending the structure of matter, the behavior of elements, and the diverse applications of atomic science in various fields. From the development of new materials to the diagnosis and treatment of diseases, the knowledge of atomic numbers continues to shape our understanding of the world and drive scientific advancements. Its importance is undeniable, serving as a cornerstone upon which much of our modern scientific understanding is built.
Latest Posts
Latest Posts
-
Where Does Mrna Go After It Leaves The Nucleus
Mar 18, 2025
-
What Is The Difference Between Primary And Secondary Growth
Mar 18, 2025
-
Work Done By An Electric Field
Mar 18, 2025
-
How Much Energy To Be At Zero Kinetic Energy
Mar 18, 2025
-
Current As A Function Of Time
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about The Number Of Protons In An Atom Is Called What . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
