The Ph Of A Solution Is
Muz Play
Mar 27, 2025 · 6 min read
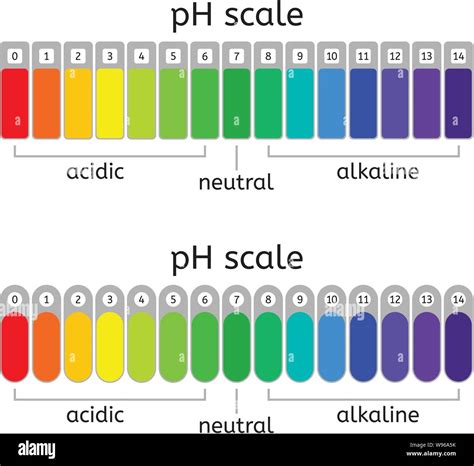
Table of Contents
The pH of a Solution Is: A Comprehensive Guide
The pH of a solution is a measure of its acidity or alkalinity. Understanding pH is crucial in various fields, from chemistry and biology to environmental science and everyday life. This comprehensive guide will delve into the intricacies of pH, exploring its definition, measurement, significance, and applications.
What is pH?
pH stands for "potential of hydrogen," a scale used to specify the acidity or basicity (alkalinity) of an aqueous solution. The scale typically ranges from 0 to 14, with 7 representing neutrality. Solutions with a pH less than 7 are considered acidic, while those with a pH greater than 7 are alkaline (basic). The pH scale is logarithmic, meaning each whole number change represents a tenfold change in the concentration of hydrogen ions (H⁺). For example, a solution with a pH of 3 is ten times more acidic than a solution with a pH of 4, and one hundred times more acidic than a solution with a pH of 5.
The Role of Hydrogen Ions (H⁺)
The pH of a solution is directly determined by the concentration of hydrogen ions (H⁺). These ions are formed when acids dissociate in water. Strong acids completely dissociate, releasing a high concentration of H⁺ ions, resulting in a low pH. Conversely, weak acids partially dissociate, releasing a lower concentration of H⁺ ions, resulting in a higher pH (though still acidic). Alkaline solutions have a low concentration of H⁺ ions and a high concentration of hydroxide ions (OH⁻). The relationship between H⁺ and OH⁻ ions is inverse; as the concentration of one increases, the concentration of the other decreases.
Measuring pH
Several methods exist for measuring the pH of a solution:
1. pH Indicators
pH indicators are substances that change color depending on the pH of the solution. These are often used in simple, qualitative pH tests. Litmus paper is a common example; it turns red in acidic solutions and blue in alkaline solutions. More sophisticated indicators, like universal indicator, provide a broader range of color changes across the pH spectrum, allowing for a more precise estimation of pH. While convenient, indicators provide only an approximate pH value.
2. pH Meters
pH meters are electronic devices that measure the pH of a solution more precisely than indicators. They typically consist of a pH-sensitive electrode (often a glass electrode) and a reference electrode. The meter measures the potential difference between these two electrodes, which is directly related to the pH of the solution. pH meters are widely used in laboratories and industries requiring accurate pH measurements. They offer high accuracy and can measure pH over a wider range compared to indicators. Regular calibration is crucial for ensuring the accuracy of pH meter readings.
3. Spectrophotometry
Spectrophotometry is a technique that measures the absorbance or transmission of light through a solution. Certain substances absorb or transmit light differently depending on the pH. By measuring the absorbance or transmission at a specific wavelength, the pH can be determined. This method is particularly useful for solutions with colored components that might interfere with other pH measurement techniques.
Significance of pH
The pH of a solution plays a crucial role in various aspects of our lives:
1. Biology and Physiology
pH is critical for the proper functioning of biological systems. The pH of human blood, for instance, is tightly regulated around 7.4. Slight deviations from this value can have severe consequences. Enzymes, the catalysts of biological reactions, function optimally within a specific pH range. Changes in pH can alter the shape and activity of enzymes, impacting metabolic processes. The pH of the digestive system also varies along its length, reflecting the different functions of each section. The stomach, for instance, has a highly acidic environment (pH around 2) to aid in digestion.
2. Chemistry and Industry
pH control is essential in many chemical processes. Many chemical reactions are pH-dependent, and precise pH control is often necessary to optimize reaction yields and product purity. Industries such as food processing, pharmaceuticals, and water treatment rely heavily on pH monitoring and control. The manufacturing of various products, including soaps, detergents, and fertilizers, requires careful pH adjustment.
3. Environmental Science
pH plays a vital role in environmental monitoring and protection. Acid rain, a result of atmospheric pollution, can significantly lower the pH of soil and water bodies, harming aquatic life and vegetation. Monitoring the pH of lakes, rivers, and oceans is crucial for assessing water quality and identifying potential pollution sources. Soil pH also significantly impacts plant growth, with different plants having different optimal pH ranges.
4. Everyday Life
pH affects many aspects of our daily lives. The pH of drinking water, for instance, is regulated to ensure safety and palatability. Many household products, such as cleaning agents and personal care items, have specific pH values tailored to their intended use. Understanding pH can help individuals make informed choices about the products they use.
Factors Affecting pH
Several factors can influence the pH of a solution:
- Temperature: Temperature changes can affect the ionization of water and thus the pH.
- Concentration of acids and bases: Higher concentrations of acids lower the pH, while higher concentrations of bases increase it.
- Presence of buffers: Buffers are solutions that resist changes in pH upon the addition of small amounts of acid or base. They play a critical role in maintaining stable pH levels in biological systems.
- Dilution: Diluting an acidic or alkaline solution will alter its pH, typically moving it closer to neutrality.
- Presence of dissolved salts: Certain salts can hydrolyze in water, affecting the pH of the solution.
Applications of pH Control
Precise pH control is essential in numerous applications:
- Water treatment: Adjusting the pH of water is vital for removing impurities and ensuring its potability.
- Agriculture: Maintaining optimal soil pH is critical for plant growth and nutrient uptake.
- Food and beverage industry: pH control is crucial in preserving food quality and preventing spoilage.
- Pharmaceutical industry: pH is carefully controlled during drug manufacturing to ensure stability and efficacy.
- Swimming pools: pH control helps maintain water clarity and prevent corrosion.
Maintaining a Constant pH: Buffers
Buffers are solutions that resist changes in pH when small amounts of acid or base are added. They consist of a weak acid and its conjugate base, or a weak base and its conjugate acid. When a small amount of acid is added to a buffer solution, the conjugate base reacts with it, minimizing the change in pH. Similarly, when a small amount of base is added, the weak acid reacts with it, again minimizing the pH change. This buffering capacity is crucial in biological systems, where maintaining a stable pH is essential for the proper functioning of enzymes and other biological molecules. The effectiveness of a buffer is described by its buffer capacity, which depends on the concentration of the buffer components and the pH relative to the pKa of the weak acid.
Conclusion
The pH of a solution is a fundamental concept with far-reaching implications in various scientific disciplines and everyday life. Understanding how pH is measured, its significance, and the factors that influence it is crucial for researchers, industrialists, and individuals alike. From maintaining the health of our bodies to ensuring the quality of our environment and the products we use, the control and monitoring of pH are essential for a healthy and sustainable world. Further research into the intricate relationships between pH and various systems will continue to yield valuable insights and applications across numerous fields.
Latest Posts
Latest Posts
-
Types Of Repeat Signs In Music
Mar 30, 2025
-
What Is Considered The Basic Unit Of Life
Mar 30, 2025
-
Determine The Force In Each Member Of Truss
Mar 30, 2025
-
Duties Of An Agent In Law Of Agency
Mar 30, 2025
-
What Is Regulating Services In Ecosystems
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about The Ph Of A Solution Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
