The Starting Components Of A Chemical Reaction Are
Muz Play
Mar 30, 2025 · 6 min read
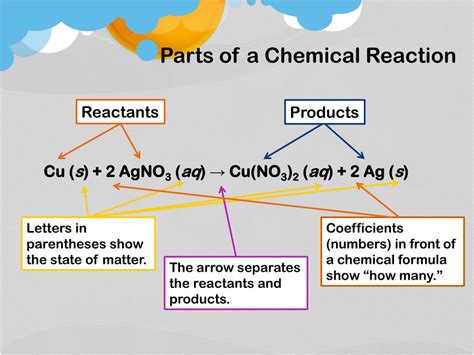
Table of Contents
The Starting Components of a Chemical Reaction: Reactants, Catalysts, and More
Chemical reactions are the fundamental processes that govern the transformations of matter. Understanding what initiates and drives these reactions is crucial in various fields, from industrial chemistry to biological systems. This article delves into the essential starting components of a chemical reaction, exploring their roles and interactions. We'll move beyond the simple notion of reactants to encompass catalysts, solvents, and other crucial players in the chemical drama unfolding before us.
Reactants: The Essential Players
The most fundamental starting components of any chemical reaction are the reactants. These are the substances that undergo a chemical change during the reaction, transforming into new substances called products. Reactants are consumed during the reaction, meaning their quantity decreases as the reaction progresses. The nature and properties of the reactants directly dictate the type of reaction that occurs and the products that are formed.
Understanding Reactant Stoichiometry
The stoichiometry of a reaction refers to the quantitative relationship between the reactants and products. It's expressed through the coefficients in a balanced chemical equation. This is crucial because it dictates the precise amounts of reactants needed to produce a specific amount of product, and to minimize waste. For example, in the reaction:
2H₂ + O₂ → 2H₂O
The stoichiometry indicates that two molecules of hydrogen gas (H₂) react with one molecule of oxygen gas (O₂) to produce two molecules of water (H₂O). This ratio is essential for efficient reaction management.
Reactant States and Phases
Reactants can exist in various states of matter: solid (s), liquid (l), gas (g), and aqueous (aq) (dissolved in water). The state of the reactants can significantly influence the reaction rate and mechanism. For instance, reactions involving solids often require higher temperatures or the presence of a solvent to increase the contact between reactant particles and thus increase the reaction rate. Gaseous reactants, on the other hand, often react more rapidly due to the higher mobility of their particles.
The Role of Reactant Concentration
The concentration of reactants, representing the amount of reactant per unit volume, is another critical factor. Higher concentrations generally lead to faster reaction rates as the probability of reactant molecules colliding and interacting increases. This is a fundamental principle exploited in various industrial processes to optimize reaction speeds and yields.
Catalysts: Accelerating the Reaction
While reactants are essential for the reaction itself, catalysts are substances that increase the rate of a chemical reaction without being consumed themselves in the process. They achieve this by providing an alternative reaction pathway with lower activation energy. This means that catalysts allow the reaction to proceed faster at a given temperature or reach completion at a lower temperature.
How Catalysts Work: Lowering Activation Energy
The activation energy is the minimum energy required for a reaction to occur. Catalysts lower this energy barrier by forming intermediate complexes with the reactants, thereby facilitating the formation of products. These catalysts remain unchanged chemically at the end of the reaction, allowing them to participate in many reaction cycles.
Types of Catalysts
Catalysts are classified into several types, including:
- Homogeneous catalysts: These catalysts exist in the same phase (solid, liquid, or gas) as the reactants. For example, a liquid catalyst dissolving in a liquid reaction mixture.
- Heterogeneous catalysts: These catalysts exist in a different phase than the reactants. A common example is a solid catalyst used in a gaseous or liquid reaction.
- Enzymes: These are biological catalysts, typically proteins, that accelerate biochemical reactions in living organisms. They are highly specific to their substrates, showcasing the incredible precision of biological catalysis.
Solvents: The Reaction Medium
Many chemical reactions, particularly those involving ionic or polar reactants, are carried out in a solvent. The solvent serves as a medium in which the reactants dissolve, increasing their contact and facilitating their interaction. The choice of solvent is often crucial, as the solvent can influence the reaction rate, selectivity, and even the outcome of the reaction.
Solvent Properties and Their Effects
The properties of the solvent, such as polarity, viscosity, and boiling point, directly impact the reaction. Polar solvents tend to dissolve polar or ionic reactants, while nonpolar solvents dissolve nonpolar reactants. The viscosity of the solvent affects the diffusion of reactants, influencing the collision frequency and reaction rate.
Solvent Selection: A Critical Consideration
Selecting the appropriate solvent is an important aspect of experimental design. The solvent should dissolve the reactants adequately, while also being inert (non-reactive) with the reactants and products. The solvent's boiling point should also be considered, as it might need to be removed after the reaction. Environmental considerations, such as toxicity and flammability, are increasingly important in solvent selection.
Inhibitors: Slowing Down the Reaction
In contrast to catalysts, inhibitors are substances that decrease the rate of a chemical reaction. They work by interfering with the reaction mechanism, often by reacting with intermediates or blocking active sites on a catalyst. Inhibitors are frequently used to control the speed of reactions, prevent unwanted side reactions, or improve product stability.
Types and Mechanisms of Inhibition
Inhibitors operate through different mechanisms, depending on the reaction and the inhibitor itself. Some inhibitors block active sites on the catalyst's surface, while others react with reactive intermediates, preventing the formation of products. The effectiveness of an inhibitor depends on factors like its concentration and the reaction conditions.
Other Important Components
Beyond reactants, catalysts, solvents, and inhibitors, other components can influence the course of a chemical reaction.
- Temperature: Increasing the temperature generally increases the reaction rate by increasing the kinetic energy of the reactants, leading to more frequent and energetic collisions.
- Pressure: Increased pressure, particularly for gaseous reactions, increases the concentration of reactants, leading to a faster reaction rate.
- Light: Some reactions are photochemically initiated, meaning they require light to start. The wavelength and intensity of the light can significantly affect the reaction rate.
- Surface Area: In heterogeneous reactions involving solids, increasing the surface area of the solid reactant can significantly enhance the reaction rate by providing more sites for reaction.
Conclusion: A Complex Interplay
The starting components of a chemical reaction are not merely isolated entities. They interact in a complex interplay, with each component influencing the reaction's rate, selectivity, and outcome. Understanding these interactions is fundamental to controlling and manipulating chemical reactions for various applications, ranging from synthesizing new materials to developing new medicines and improving industrial processes. By carefully choosing and controlling the starting components, chemists can optimize reactions to achieve desired products efficiently and safely. Furthermore, ongoing research continuously expands our understanding of these intricate processes, revealing new ways to design and control chemical transformations. The study of reactants, catalysts, solvents, and other factors remains at the heart of chemical research, continuously driving innovation and progress.
Latest Posts
Latest Posts
-
Collection Of Neuron Cell Bodies Found Within The Cns
Apr 01, 2025
-
An Atom That Loses An Electron Is Called
Apr 01, 2025
-
Are Daughter Cells Identical To Parent Cells In Mitosis
Apr 01, 2025
-
Hair Like Outgrowths That Attach To Bacteria
Apr 01, 2025
-
Which Of The Following Is An Example Of Ottonian Architecture
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about The Starting Components Of A Chemical Reaction Are . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
