An Atom That Loses An Electron Is Called
Muz Play
Apr 01, 2025 · 6 min read
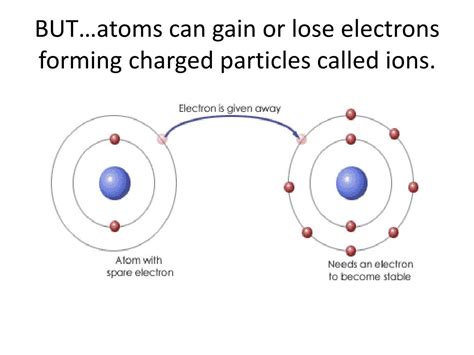
Table of Contents
An Atom That Loses an Electron is Called an Ion: A Deep Dive into Ionization
When an atom loses an electron, it's no longer electrically neutral. This crucial change transforms it into a ion, specifically a cation. Understanding ionization is fundamental to comprehending chemistry, physics, and numerous technological applications. This article will delve deep into the process of ionization, exploring its causes, consequences, and significance across various scientific disciplines.
What is Ionization?
Ionization is the process by which an atom or molecule acquires a net electrical charge by gaining or losing electrons. The loss of electrons results in a positive ion, or cation, while the gain of electrons leads to a negative ion, or anion. The magnitude of the charge depends on the number of electrons gained or lost. For example, losing one electron creates a +1 ion, losing two creates a +2 ion, and so on.
Key takeaway: The core concept is the imbalance of protons and electrons. A neutral atom has an equal number of protons (positive charge) and electrons (negative charge). Ionization disrupts this balance, leading to a charged particle.
The Role of Electrons in Ionization
Electrons are fundamental particles orbiting the nucleus of an atom. They're held in place by the electrostatic attraction to the positively charged protons in the nucleus. However, this attraction is not insurmountable. External forces can overcome the electrostatic pull, leading to the ejection of electrons and the formation of ions.
Factors Affecting Ionization
Several factors influence the likelihood of ionization:
-
Electromagnetic radiation: High-energy photons, like those found in X-rays and gamma rays, possess enough energy to knock electrons out of atoms. This is a common method used in various analytical techniques.
-
Collisions: Atoms can be ionized through collisions with other particles, especially high-energy particles like electrons or alpha particles. This process is prevalent in plasma environments and particle accelerators.
-
Chemical reactions: Some chemical reactions involve the transfer of electrons between atoms. In these reactions, one atom may lose electrons, becoming a cation, while another gains electrons, becoming an anion. This is the basis of ionic bonding.
-
Temperature: High temperatures provide atoms with sufficient kinetic energy to overcome the electrostatic attraction holding their electrons. This is why ionization is common in stars and other high-temperature environments.
Types of Ions: Cations and Anions
As mentioned earlier, ionization results in two primary types of ions:
Cations: Positively Charged Ions
A cation is formed when an atom loses one or more electrons. The loss of negatively charged electrons leaves the atom with a net positive charge. The number of positive charges equals the number of electrons lost. For example:
- Sodium (Na) losing one electron becomes Na⁺ (sodium cation).
- Calcium (Ca) losing two electrons becomes Ca²⁺ (calcium cation).
- Aluminum (Al) losing three electrons becomes Al³⁺ (aluminum cation).
The tendency of an atom to lose electrons and form a cation is related to its position in the periodic table. Atoms with low ionization energies (the energy required to remove an electron) readily lose electrons and form cations. These are typically metals located on the left-hand side of the periodic table.
Anions: Negatively Charged Ions
An anion is formed when an atom gains one or more electrons. The addition of negatively charged electrons gives the atom a net negative charge. The number of negative charges equals the number of electrons gained. For example:
- Chlorine (Cl) gaining one electron becomes Cl⁻ (chloride anion).
- Oxygen (O) gaining two electrons becomes O²⁻ (oxide anion).
- Nitrogen (N) gaining three electrons becomes N³⁻ (nitride anion).
Atoms with high electron affinities (the energy change associated with gaining an electron) readily gain electrons and form anions. These are typically nonmetals located on the right-hand side of the periodic table.
The Significance of Ionization
Ionization plays a vital role in various areas:
1. Chemistry: Ionic Bonding and Chemical Reactions
Ionic bonding, a fundamental type of chemical bond, is formed through the electrostatic attraction between cations and anions. This type of bonding is responsible for the formation of many ionic compounds, which are essential components in various materials and biological systems. Understanding ionization is crucial for predicting and explaining chemical reactions involving electron transfer.
2. Physics: Plasma Physics and Astrophysics
Plasma, often called the fourth state of matter, is a highly ionized gas consisting of free electrons and ions. Plasma physics is crucial in understanding stars, fusion reactions, and various technological applications, including plasma displays and plasma etching in semiconductor manufacturing. Ionization processes are fundamental to understanding the behavior of matter under extreme conditions found in stars and other celestial bodies.
3. Biology: Biological Processes and Medical Applications
Ionization plays a critical role in several biological processes. For instance, the transmission of nerve impulses relies on the movement of ions across cell membranes. Furthermore, ionization is employed in various medical applications, including radiation therapy for cancer treatment and ionization detectors for medical imaging.
4. Technology: Semiconductor Technology and Mass Spectrometry
Ionization is central to many technological advancements. In semiconductor manufacturing, techniques like plasma etching utilize ionized gases to precisely remove material from silicon wafers. Mass spectrometry, a powerful analytical technique used to identify and quantify molecules, relies on ionizing molecules to measure their mass-to-charge ratio.
Ionization Energy and its Relation to Periodic Trends
Ionization energy is the minimum energy required to remove an electron from a neutral gaseous atom in its ground state. This energy is a crucial property that reflects the atom's tendency to lose electrons. Ionization energy exhibits distinct periodic trends:
-
Across a period (left to right): Ionization energy generally increases. This is because the effective nuclear charge (the net positive charge experienced by valence electrons) increases, making it harder to remove electrons.
-
Down a group (top to bottom): Ionization energy generally decreases. This is because the atomic radius increases, and the valence electrons are further away from the nucleus, experiencing weaker electrostatic attraction.
Detecting Ions: Methods and Techniques
Various methods are employed to detect and analyze ions:
-
Mass spectrometry: This technique separates ions based on their mass-to-charge ratio, enabling the identification and quantification of different ions in a sample.
-
Spectroscopy: Spectroscopic techniques, such as atomic emission spectroscopy and atomic absorption spectroscopy, analyze the light emitted or absorbed by ions, providing information about their electronic structure and concentration.
-
Electrochemical methods: These methods measure the movement of ions in solutions, providing information about their concentration and mobility. Examples include potentiometry and voltammetry.
Conclusion: The Ubiquitous Nature of Ions
An atom that loses an electron is called a cation, a type of ion. Ionization, the process leading to the formation of ions, is a fundamental phenomenon with far-reaching implications across various scientific fields and technological applications. Understanding the causes, consequences, and significance of ionization is critical for comprehending the behavior of matter at the atomic and molecular levels, and for developing new technologies based on the properties of ions. From the chemical bonds that hold molecules together to the workings of stars and the development of advanced technologies, ions play a pivotal role in shaping our world. The continued study and application of ionization knowledge will undoubtedly lead to further breakthroughs in science and technology.
Latest Posts
Latest Posts
-
Liquids Have A Definite Shape And Volume
Apr 02, 2025
-
Difference Between Molar Mass And Atomic Mass
Apr 02, 2025
-
Write In Standard Form The Equation Of Each Line
Apr 02, 2025
-
What Does Fad Stand For In Biology
Apr 02, 2025
-
The Outermost Layer Of The Heart Is Called The
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about An Atom That Loses An Electron Is Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
