This Type Of Reaction Requires Energy In Order To Proceed
Muz Play
Mar 21, 2025 · 6 min read
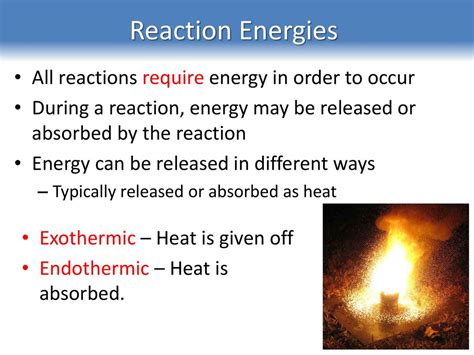
Table of Contents
Endothermic Reactions: When Energy Input Fuels Chemical Change
Endothermic reactions are fascinating processes in chemistry where the system absorbs energy from its surroundings to proceed. Unlike their counterparts, exothermic reactions, which release energy, endothermic reactions require a constant input of energy to drive the reaction forward. This energy can take various forms, including heat, light, or electricity. Understanding the intricacies of endothermic reactions is crucial in various fields, from industrial processes to biological systems. This comprehensive guide delves deep into the mechanisms, characteristics, and real-world applications of these energy-demanding reactions.
Understanding the Fundamentals of Endothermic Reactions
At the heart of every endothermic reaction lies the concept of enthalpy (ΔH). Enthalpy represents the total heat content of a system. In endothermic reactions, the enthalpy of the products is higher than the enthalpy of the reactants. This means the system gains energy during the reaction, resulting in a positive change in enthalpy (ΔH > 0). This positive ΔH is a hallmark of an endothermic process.
Visualizing Energy Changes
Imagine a graph depicting the energy changes during a reaction. For an endothermic reaction, the energy level of the products is higher than the energy level of the reactants. The difference between these energy levels represents the energy absorbed by the system. This energy is often supplied in the form of heat, causing a decrease in the temperature of the surroundings. Think of it like a sponge absorbing water; the sponge (the system) gains energy (the water), causing a decrease in the available water (energy) in the environment.
The Activation Energy Barrier
Even though endothermic reactions absorb energy overall, they still need to overcome an initial energy barrier known as the activation energy. This activation energy is the minimum amount of energy required to initiate the reaction. It's like pushing a rock uphill; you need to put in some initial effort (activation energy) before the rock starts rolling downhill (the reaction proceeds). Once the activation energy is surpassed, the reaction can proceed, absorbing energy from its surroundings as it progresses.
Characteristics of Endothermic Reactions
Several key characteristics distinguish endothermic reactions from their exothermic counterparts:
-
Positive Enthalpy Change (ΔH > 0): This is the most defining characteristic. The system gains energy, resulting in a positive value for the change in enthalpy.
-
Temperature Decrease: Because the reaction absorbs heat from its surroundings, the temperature of the immediate environment typically decreases. You might feel a cooling effect during an endothermic process.
-
Energy Absorbing: The reaction continuously absorbs energy throughout its course. This energy input is essential for the reaction to proceed.
-
Often Non-Spontaneous: Many endothermic reactions are non-spontaneous, meaning they don't occur naturally without an external energy input. They require a continuous supply of energy to drive the reaction forward.
-
Examples include: Photosynthesis (plants absorbing light energy), melting ice (absorbing heat energy), and the evaporation of water (absorbing heat energy).
Common Examples of Endothermic Reactions in Daily Life and Industry
Endothermic reactions aren't just theoretical concepts; they play a significant role in our daily lives and various industrial processes:
1. Photosynthesis: The Engine of Life
Photosynthesis, the process by which plants convert light energy into chemical energy in the form of glucose, is a prime example of an endothermic reaction. Plants absorb sunlight (energy) to convert carbon dioxide and water into glucose and oxygen. This process is fundamental to sustaining life on Earth.
2. Melting Ice: A Phase Transition
The melting of ice is a classic example of an endothermic process. Ice absorbs heat from its surroundings to overcome the intermolecular forces holding its molecules together in a solid state, transitioning into liquid water. The temperature remains constant at 0°C (32°F) during the melting process until all the ice has melted.
3. Cooking an Egg: Denaturation and Absorption
Cooking an egg involves several endothermic reactions. The heat from the cooking process causes the proteins in the egg white and yolk to unfold and change their structure (denaturation), a process that absorbs energy.
4. Evaporation of Water: Cooling Effect
The evaporation of water is another endothermic process. Liquid water absorbs heat energy from its surroundings to overcome the intermolecular forces and transform into water vapor. This is why sweating cools the body; the evaporation of sweat absorbs heat from the skin.
5. Dissolving Salts: Endothermic Dissolution
Dissolving certain salts in water is an endothermic process. The process absorbs energy from the surroundings, resulting in a decrease in temperature. You might notice a cooling sensation when dissolving certain salts, like ammonium nitrate, in water.
6. Industrial Processes: Endothermic Reactions in Manufacturing
Numerous industrial processes rely on endothermic reactions. These include the production of certain chemicals, the refining of metals, and various other manufacturing processes that require substantial energy inputs.
Factors Affecting Endothermic Reactions
Several factors influence the rate and extent of endothermic reactions:
1. Temperature: The Energy Supply
Increasing the temperature provides more energy to the system, increasing the reaction rate. Higher temperatures increase the kinetic energy of the reactant molecules, making them more likely to overcome the activation energy barrier.
2. Concentration: Reactant Availability
Increasing the concentration of reactants increases the frequency of collisions between molecules, thus increasing the reaction rate. More reactants mean more opportunities for the reaction to occur.
3. Surface Area: Accessibility of Reactants
For reactions involving solids, increasing the surface area of the reactants increases the reaction rate. A larger surface area allows for more frequent collisions between reactant molecules.
4. Catalysts: Lowering the Activation Energy
Catalysts are substances that increase the rate of a reaction without being consumed themselves. They do this by lowering the activation energy, making it easier for the reaction to proceed. While catalysts don't change the overall enthalpy change, they significantly speed up the reaction.
Applications of Endothermic Reactions in Different Fields
The principles of endothermic reactions have widespread applications across diverse fields:
1. Refrigeration and Air Conditioning: Harnessing the Cooling Effect
The cooling effect of endothermic processes is harnessed in refrigeration and air conditioning systems. These systems utilize refrigerants that absorb heat from the surroundings, cooling the environment.
2. Medicine: Targeted Drug Delivery
Endothermic reactions play a role in some drug delivery systems. Certain formulations utilize endothermic processes to control the release of drugs in the body.
3. Environmental Science: Understanding Climate Change
Understanding endothermic and exothermic processes is crucial in comprehending climate change and its effects. The absorption and release of heat in various natural processes play a significant role in the Earth's climate system.
4. Materials Science: Designing New Materials
Knowledge of endothermic reactions is applied in materials science to design and synthesize new materials with specific properties. The energy requirements of different reactions are taken into account when developing new materials.
Conclusion: The Importance of Endothermic Reactions
Endothermic reactions, while requiring an external energy input, are essential for a wide range of natural and industrial processes. From the fundamental processes of life like photosynthesis to technological advancements like refrigeration, understanding these energy-absorbing reactions is crucial. Their characteristics, influencing factors, and applications continue to be studied and applied in various scientific and technological fields, driving innovation and expanding our knowledge of the natural world. Further research into endothermic processes continues to unlock new possibilities in diverse areas, contributing to advancements in various scientific disciplines and technological applications.
Latest Posts
Latest Posts
-
How To Calculate Safe Dose Range
Mar 21, 2025
-
Do Individual Charged Particles Have Magnetic Fields
Mar 21, 2025
-
How Many Electrons Does P Have
Mar 21, 2025
-
Mannitol Salt Agar Is Selective For
Mar 21, 2025
-
What Is The Cleft Of Venus
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about This Type Of Reaction Requires Energy In Order To Proceed . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
