To Decrease The Concentration Of A Solution Add More Liquid
Muz Play
Mar 25, 2025 · 6 min read
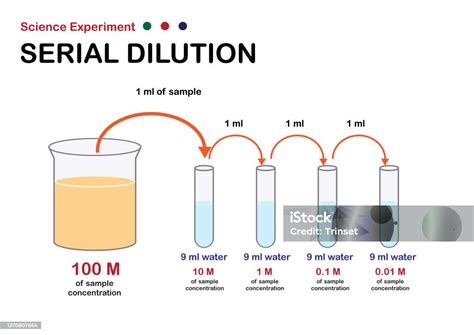
Table of Contents
Diluting Solutions: A Comprehensive Guide to Decreasing Concentration by Adding More Liquid
Adding more liquid to a solution is a fundamental technique in chemistry and various other fields to decrease the concentration of a solute. This process, known as dilution, is crucial for preparing solutions of specific concentrations, adjusting reaction conditions, and numerous other applications. Understanding the principles behind dilution, the methods employed, and the potential challenges involved is essential for accurate and safe laboratory practices and various industrial processes. This comprehensive guide delves into the intricacies of dilution, providing a detailed explanation of the process, its applications, and best practices.
Understanding Concentration and Dilution
Before diving into the mechanics of dilution, let's clarify the concept of concentration. Concentration refers to the amount of solute dissolved in a given amount of solvent or solution. It can be expressed in various ways, including molarity (moles of solute per liter of solution), molality (moles of solute per kilogram of solvent), percent concentration (by mass or volume), and parts per million (ppm).
Dilution, conversely, is the process of decreasing the concentration of a solution by adding more solvent. This doesn't change the amount of solute; it simply increases the total volume, thus reducing the solute's concentration per unit volume. The fundamental principle behind dilution is the conservation of the amount of solute. The number of moles of solute remains constant during the dilution process.
The Mathematics of Dilution: Calculating Desired Concentrations
Accurately diluting a solution requires precise calculations. The most common method involves using the following formula, based on the principle of conservation of moles:
M1V1 = M2V2
Where:
- M1 is the initial concentration of the solution.
- V1 is the initial volume of the solution.
- M2 is the final concentration of the diluted solution.
- V2 is the final volume of the diluted solution.
This formula allows you to calculate any of the four variables if you know the other three. For example, you can determine the volume of stock solution needed to prepare a specific volume of a diluted solution with a known concentration. It's crucial to use consistent units throughout the calculation (e.g., liters for volume and molarity for concentration).
Example Calculation:
Let's say you have a 2.0 M stock solution of hydrochloric acid (HCl) and you need to prepare 500 mL of a 0.1 M HCl solution. Using the formula:
M1V1 = M2V2
(2.0 M)(V1) = (0.1 M)(500 mL)
Solving for V1:
V1 = (0.1 M * 500 mL) / 2.0 M = 25 mL
Therefore, you would need to measure 25 mL of the 2.0 M HCl stock solution and add enough solvent (usually distilled water) to bring the total volume to 500 mL.
Practical Techniques for Diluting Solutions
The actual process of diluting a solution involves several crucial steps to ensure accuracy and safety.
1. Safety First: Appropriate Personal Protective Equipment (PPE)
Always wear appropriate PPE, including safety goggles, gloves, and a lab coat, when handling chemicals. Some solutions can be corrosive or harmful, so proper safety precautions are paramount.
2. Using Appropriate Glassware: Volumetric Flasks and Pipettes
Accurate dilution relies on precise volume measurements. Volumetric flasks are designed to contain a specific volume with high accuracy, and are ideal for preparing diluted solutions. Graduated pipettes or volumetric pipettes are used for accurately transferring the required volume of the stock solution. Avoid using beakers or Erlenmeyer flasks for precise volume measurements, as they are less accurate.
3. The Dilution Process: Step-by-Step
- Calculate the required volume: Use the M1V1 = M2V2 formula to determine the volume of stock solution needed.
- Add a portion of the solvent: Add a small amount of the solvent (usually distilled water) to the volumetric flask, about half its final volume. This ensures efficient mixing later.
- Transfer the stock solution: Carefully transfer the calculated volume of the stock solution to the volumetric flask using the appropriate pipette. Rinse the pipette with a small amount of solvent and add the rinse to the flask to ensure complete transfer of the solute.
- Add more solvent: Add more solvent to the flask until the bottom of the meniscus aligns with the calibration mark on the neck of the volumetric flask.
- Mix thoroughly: Stopper the flask and invert it several times to ensure the solution is thoroughly mixed.
Applications of Dilution in Various Fields
Dilution is a ubiquitous technique used across diverse fields:
1. Analytical Chemistry: Preparing Standard Solutions
In analytical chemistry, preparing solutions of known concentrations, called standard solutions, is crucial for various analytical techniques such as titration, spectrophotometry, and chromatography. These solutions are typically prepared by diluting a more concentrated stock solution.
2. Clinical Chemistry and Medicine: Drug Preparation and Administration
In healthcare, accurate dilution is crucial for preparing medications and intravenous solutions. The concentration of drugs administered to patients must be precisely controlled to ensure safety and efficacy.
3. Environmental Science: Sample Preparation and Analysis
Environmental samples, such as water or soil, often require dilution before analysis to bring the analyte concentration within the measurable range of the analytical instrument.
4. Brewing and Food Science: Adjusting Flavor and Concentration
Dilution plays a critical role in adjusting the concentration of ingredients in various food and beverage preparations. For example, diluting fruit juices or concentrating syrups is common practice.
5. Industrial Processes: Chemical Reactions and Cleaning
In industrial processes, dilution is frequently used to control the concentration of reactants in chemical reactions or to dilute cleaning solutions.
Potential Challenges and Considerations in Dilution
While dilution is a relatively straightforward process, certain challenges and considerations must be addressed:
1. Heat of Dilution: Exothermic and Endothermic Reactions
Some solutions exhibit a significant heat of dilution, either releasing (exothermic) or absorbing (endothermic) heat upon dilution. Exothermic reactions can cause the solution to become hot, possibly leading to boiling or splashing. Always add the concentrated solution to the solvent slowly and cautiously, especially when diluting highly concentrated acids or bases.
2. Incomplete Mixing: Ensuring Homogeneity
Incomplete mixing can lead to inaccurate concentrations in different parts of the solution. Thorough mixing is crucial to ensure homogeneity. Using a magnetic stirrer with a stir bar is highly recommended for larger volumes.
3. Using the Correct Solvent: Solubility and Interactions
The choice of solvent is critical. The solvent should be compatible with the solute and capable of dissolving it completely. Using the wrong solvent may lead to precipitation or other unwanted reactions.
4. Volumetric Accuracy: Precision and Error
Precise measurements are essential. Using calibrated glassware and employing careful techniques minimizes errors in volume measurements. The accuracy of the final concentration directly depends on the accuracy of the volume measurements.
5. Serial Dilutions: Preparing Very Dilute Solutions
For preparing very dilute solutions, it's often more accurate to perform a series of dilutions rather than one large dilution. Serial dilutions reduce the risk of errors associated with measuring extremely small volumes.
Conclusion: Mastering the Art of Dilution
Dilution is a fundamental technique with far-reaching applications across diverse scientific and industrial fields. Understanding the underlying principles, mastering the necessary calculations, and employing proper techniques are essential for accurate and safe dilution procedures. By adhering to safety protocols, using appropriate glassware, and paying close attention to detail, you can ensure the reliable preparation of solutions with precisely controlled concentrations, paving the way for accurate experimental results and efficient processes. Consistent practice and careful attention to detail are key to mastering the art of dilution and ensuring successful outcomes in any application.
Latest Posts
Latest Posts
-
Gramatica A The Verb Gustar Worksheet Answers
Mar 26, 2025
-
What Is The Vertical Columns On The Periodic Table Called
Mar 26, 2025
-
Nonpolar Molecules Are The Result Of Unequal Electron Pair Sharing
Mar 26, 2025
-
What Does Triangle Mean In Physics
Mar 26, 2025
-
Moment Of Inertia For A Uniform Rod
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about To Decrease The Concentration Of A Solution Add More Liquid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
