What Is The Vertical Columns On The Periodic Table Called
Muz Play
Mar 26, 2025 · 6 min read
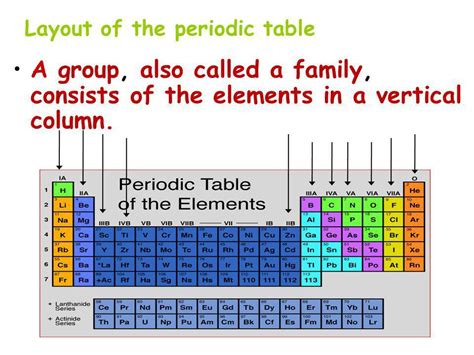
Table of Contents
What are the Vertical Columns on the Periodic Table Called? A Deep Dive into Groups and Families
The periodic table, that iconic chart of chemical elements, is more than just a neatly organized list. Its structure, with its horizontal rows and vertical columns, reveals fundamental relationships between elements, predicting their properties and behaviors. While everyone knows the horizontal rows are called periods, many are less familiar with the names and significance of the vertical columns. This article will delve into the answer to the question: what are the vertical columns on the periodic table called? We'll explore their nomenclature, the underlying principles of their organization, and the crucial role they play in chemistry.
Understanding the Vertical Columns: Groups and Families
The vertical columns on the periodic table are called groups or families. These terms are often used interchangeably, and both accurately describe these columns. The elements within each group share similar chemical properties because they have the same number of valence electrons. This is the key to understanding why elements in the same group behave similarly.
Valence Electrons: The Key to Group Similarity
Valence electrons are the electrons located in the outermost shell (energy level) of an atom. These electrons are the ones involved in chemical bonding, dictating how an atom will interact with other atoms to form molecules and compounds. Elements within the same group possess the same number of valence electrons. This shared characteristic leads to strikingly similar chemical behaviors. For example, elements in Group 1 (the alkali metals) all have one valence electron, leading to their high reactivity and tendency to lose that electron to form +1 ions.
Group Numbering: A System of Classification
The numbering system for groups has evolved over time. You may encounter both the older, Roman numeral system (IA, IIA, IIIA, etc.) and the newer, Arabic numeral system (1, 2, 13, etc.). While both systems refer to the same groups, the newer system is more widely accepted today. The Arabic numbering system runs from 1 to 18, encompassing all the groups.
Exploring the Major Groups and Their Characteristics
Let's explore some of the key groups on the periodic table, highlighting their defining properties and representative elements:
Group 1: The Alkali Metals
This group is characterized by its high reactivity. Alkali metals have one valence electron, readily lost to form +1 ions. This makes them extremely reactive with water and air. Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Cesium (Cs), and Francium (Fr) are the members of this group. Their reactivity increases as you go down the group.
Key Properties:
- Highly reactive: React vigorously with water, often producing flames.
- Soft metals: Can be easily cut with a knife.
- Low densities: Generally less dense than other metals.
- Form +1 ions: Easily lose one electron to achieve a stable electron configuration.
Group 2: The Alkaline Earth Metals
Similar to alkali metals, alkaline earth metals are also reactive, although less so than their Group 1 counterparts. They have two valence electrons, readily losing them to form +2 ions. Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba), and Radium (Ra) constitute this group.
Key Properties:
- Reactive (less than alkali metals): React with water, though often less vigorously than alkali metals.
- Relatively hard: Harder than alkali metals.
- Higher densities: Denser than alkali metals.
- Form +2 ions: Easily lose two electrons to achieve a stable electron configuration.
Group 17: The Halogens
Halogens are highly reactive nonmetals. They have seven valence electrons, readily gaining one electron to form -1 ions. This strong tendency to gain an electron contributes to their high reactivity. Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I), and Astatine (At) make up this group.
Key Properties:
- Highly reactive nonmetals: React readily with many metals and other nonmetals.
- Form -1 ions: Easily gain one electron to achieve a stable electron configuration.
- Diatomic molecules: Exist as diatomic molecules (e.g., F₂, Cl₂) in their elemental form.
- Varied physical states: Fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid at room temperature.
Group 18: The Noble Gases
Noble gases are unique in their extreme unreactivity. They have a full valence shell of eight electrons (except for helium, which has two), making them extremely stable and resistant to forming chemical bonds. This explains their inert nature. Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), and Radon (Rn) are included in this group.
Key Properties:
- Inert: Extremely unreactive due to their full valence electron shells.
- Gases at room temperature: Exist as monatomic gases under normal conditions.
- Used in various applications: Helium is used in balloons and cryogenics, while other noble gases find use in lighting and other technologies.
Transition Metals: A Special Case
The central region of the periodic table houses the transition metals. These elements differ from the main group elements (Groups 1-2 and 13-18) in their electronic configuration. Transition metals have partially filled d orbitals, which contribute to their diverse chemical properties and ability to form multiple oxidation states. This explains their varied colors and catalytic abilities.
Key Characteristics of Transition Metals:
- Variable oxidation states: Can exist in multiple oxidation states, leading to a wide range of compounds.
- Colorful compounds: Often form brightly colored compounds due to the d-electron transitions.
- Catalytic activity: Many transition metals act as catalysts in chemical reactions.
- High melting and boiling points: Generally have high melting and boiling points compared to main group elements.
Inner Transition Metals: Lanthanides and Actinides
Below the main body of the periodic table are two rows of elements: the lanthanides and the actinides. These elements are also known as the inner transition metals. They are placed separately to maintain the overall table’s structure and readability. Both series have partially filled f orbitals, leading to similar chemical properties within each series.
- Lanthanides: These elements follow lanthanum (La) and show similar chemical properties.
- Actinides: These elements follow actinium (Ac) and are mostly radioactive.
The Importance of Group Organization
The organization of the periodic table by groups (vertical columns) is incredibly important for several reasons:
- Predicting properties: Knowing the group an element belongs to allows chemists to predict its chemical behavior and properties.
- Understanding reactivity: The number of valence electrons determines an element's reactivity, which is directly related to its group placement.
- Designing new materials: Understanding group properties helps in the design and synthesis of new materials with specific characteristics.
- Developing chemical theories: The periodic table and its group organization are fundamental to our understanding of chemical bonding and chemical reactions.
Conclusion: Groups – The Foundation of Chemical Understanding
The vertical columns on the periodic table, known as groups or families, are far more than just a convenient organizational tool. They represent a fundamental aspect of chemical behavior, reflecting the underlying electronic structure of the elements. Understanding the characteristics of each group, their valence electron configurations, and the resulting chemical properties provides a crucial framework for predicting and interpreting chemical phenomena, furthering the advancement of chemistry as a whole. From the highly reactive alkali metals to the inert noble gases, the groups on the periodic table offer a rich tapestry of chemical diversity and interconnectedness. The systematic arrangement of elements based on their group affiliation is essential for our understanding of the chemical world and continues to be an invaluable tool for chemists worldwide.
Latest Posts
Latest Posts
-
In Cellular Respiration Most Atp Molecules Are Produced By
Mar 29, 2025
-
What Is The Power Stroke In Muscle Contraction
Mar 29, 2025
-
What Is The Molecular Ion Peak
Mar 29, 2025
-
Genomics Can Be Used In Agriculture To
Mar 29, 2025
-
Standard Enthalpy Of Formation For O2
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Is The Vertical Columns On The Periodic Table Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
