True Or False Osmosis Is A Type Of Diffusion
Muz Play
Mar 19, 2025 · 6 min read
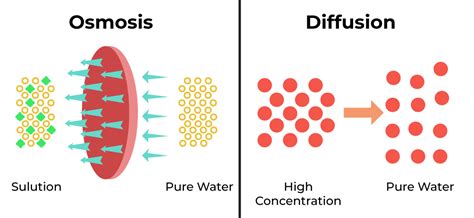
Table of Contents
True or False: Osmosis is a Type of Diffusion?
The statement "Osmosis is a type of diffusion" is true. However, understanding why this is true requires a deeper dive into the mechanisms of both osmosis and diffusion. This article will thoroughly explore the relationship between these two crucial biological processes, clarifying their similarities and highlighting their key differences. We'll unravel the complexities, demystify the terminology, and ultimately solidify your understanding of this fundamental concept in biology.
Understanding Diffusion: The Movement of Particles
Diffusion is the net passive movement of particles from a region of higher concentration to a region of lower concentration. This movement continues until the particles are evenly distributed throughout the available space. This process doesn't require energy input; it's driven by the inherent kinetic energy of the particles themselves—they're constantly in motion, colliding and bouncing off each other. Think of a drop of ink spreading out in a glass of water: the ink particles diffuse until they're uniformly distributed throughout the water.
Several factors influence the rate of diffusion:
- Concentration gradient: A steeper concentration gradient (a larger difference in concentration between two regions) leads to faster diffusion.
- Temperature: Higher temperatures increase the kinetic energy of particles, resulting in faster diffusion.
- Mass of the particles: Smaller particles diffuse faster than larger ones.
- Distance: Diffusion is slower over longer distances.
- Surface area: A larger surface area allows for faster diffusion.
- Medium: The medium through which diffusion occurs affects the rate; diffusion is generally faster in gases than in liquids, and slower in solids.
Types of Diffusion: Simple and Facilitated
While the basic principle remains consistent, diffusion can be classified into two main types:
-
Simple diffusion: This involves the movement of particles directly across the cell membrane without the assistance of any membrane proteins. Small, nonpolar molecules like oxygen and carbon dioxide readily diffuse across the lipid bilayer of the cell membrane.
-
Facilitated diffusion: This type of diffusion relies on membrane proteins to help transport particles across the membrane. Larger or polar molecules that cannot easily cross the lipid bilayer use specific protein channels or carrier proteins to facilitate their movement. This process is still passive, meaning it doesn't require energy, but it is facilitated by the proteins.
Osmosis: Diffusion of Water Across a Selectively Permeable Membrane
Osmosis is a special case of diffusion. It's defined as the net movement of water molecules across a selectively permeable membrane from a region of higher water concentration to a region of lower water concentration. The key difference here is the presence of a selectively permeable membrane, which allows some substances to pass through but restricts the passage of others. This membrane is often a cell membrane, but it can also be an artificial membrane like dialysis tubing.
The concentration of water is inversely related to the concentration of solutes (dissolved substances). A solution with a high concentration of solutes has a lower concentration of water, and vice versa. Water moves to dilute the region with a higher solute concentration, trying to equalize the solute concentration on both sides of the membrane.
Understanding Osmotic Pressure
Osmotic pressure is the pressure that must be applied to prevent the inward flow of water across a selectively permeable membrane. It's a measure of the tendency of water to move into a solution. A solution with a high solute concentration (and thus a low water concentration) will exert a higher osmotic pressure than a solution with a low solute concentration.
Tonicity: Describing Osmotic Environments
The tonicity of a solution describes its effect on the volume of a cell placed in that solution. There are three main types of tonicity:
-
Isotonic solution: The solute concentration is equal inside and outside the cell. There is no net movement of water, and the cell maintains its normal shape.
-
Hypotonic solution: The solute concentration is lower outside the cell than inside. Water moves into the cell, causing it to swell and potentially burst (lyse) in animal cells. Plant cells, however, have a cell wall that prevents bursting; instead, they become turgid (firm).
-
Hypertonic solution: The solute concentration is higher outside the cell than inside. Water moves out of the cell, causing it to shrink (crenate) in animal cells. Plant cells undergo plasmolysis, where the cell membrane pulls away from the cell wall.
Osmosis and Diffusion: Key Similarities and Differences
Both osmosis and diffusion are passive transport processes that don't require energy. They both involve the movement of particles from a region of higher concentration to a region of lower concentration, aiming to reach equilibrium.
However, the key difference lies in what is moving and the presence of a selectively permeable membrane:
| Feature | Diffusion | Osmosis |
|---|---|---|
| Movement of | Any particles | Primarily water molecules |
| Membrane | May or may not involve a membrane | Requires a selectively permeable membrane |
| Specificity | Non-specific; all particles move | Specific; only water moves across the membrane |
| Equilibrium | Equal distribution of particles | Equal water potential across the membrane |
The Importance of Osmosis in Biological Systems
Osmosis plays a vital role in many biological processes:
-
Water uptake in plants: Osmosis drives the movement of water from the soil into plant roots, allowing plants to obtain essential nutrients and maintain turgor pressure.
-
Nutrient absorption in the gut: Osmosis plays a part in the absorption of water and nutrients from the digested food in the intestines.
-
Regulation of blood pressure: Osmosis helps maintain the proper balance of fluids in the bloodstream, contributing to blood pressure regulation.
-
Kidney function: The kidneys use osmosis to filter waste products from the blood and regulate the body's water balance.
-
Cell shape and volume: Osmosis ensures that cells maintain their appropriate volume and shape by controlling the water balance within them.
Real-World Applications and Examples
Understanding osmosis has far-reaching implications in various fields:
-
Medicine: Dialysis relies on osmosis to remove waste products from the blood of patients with kidney failure. Intravenous fluids are carefully formulated to be isotonic to prevent damage to blood cells.
-
Agriculture: Osmosis plays a critical role in irrigation and fertilizer uptake by plants. Understanding osmotic pressure is essential for optimizing water and nutrient delivery to crops.
-
Food preservation: Osmosis is used in food preservation techniques like pickling, where high solute concentrations prevent bacterial growth by drawing water out of the food.
-
Water purification: Reverse osmosis is a technology used to purify water by forcing it through a semi-permeable membrane, removing impurities.
Conclusion: Osmosis is a Specialized Form of Diffusion
In conclusion, the statement "Osmosis is a type of diffusion" is unequivocally true. Osmosis is a specialized form of diffusion that specifically addresses the passive movement of water across a selectively permeable membrane. While both processes aim to achieve equilibrium by moving particles from high to low concentration, osmosis focuses on water movement driven by differences in water potential or solute concentration across a membrane. Understanding this distinction is crucial for comprehending many fundamental biological processes and their broader applications in various fields. The intricacies of osmosis and its interplay with diffusion demonstrate the elegance and efficiency of biological systems.
Latest Posts
Latest Posts
-
What Is Monomer Of Nucleic Acids
Mar 19, 2025
-
Inverted Vs Everted Palindromic Dna Sequence Example
Mar 19, 2025
-
What Makes Up Most Of The Mass Of An Atom
Mar 19, 2025
-
Policy Implementation Refers To The Bureaucratic Function Of
Mar 19, 2025
-
Example Of A Formal Lab Report For Chemistry
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about True Or False Osmosis Is A Type Of Diffusion . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
