Understanding The Definitions Of Ionization Energy And Electron Affinity
Muz Play
Mar 17, 2025 · 6 min read
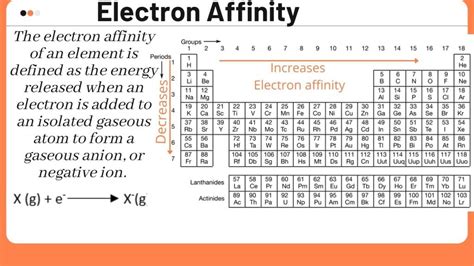
Table of Contents
Understanding the Definitions of Ionization Energy and Electron Affinity
Understanding the behavior of atoms and their interactions is fundamental to chemistry. Two crucial concepts in this understanding are ionization energy and electron affinity. While seemingly simple at first glance, these properties reveal much about an atom's electronic structure and its propensity to participate in chemical reactions. This article delves deep into the definitions, trends, factors influencing these properties, and their practical applications.
Ionization Energy: The Energy Cost of Removing an Electron
Ionization energy (IE), also known as ionization potential, is the minimum amount of energy required to remove the most loosely bound electron from a neutral gaseous atom or ion. This process creates a positively charged ion, or cation. It's crucial to note that the atom must be in the gaseous state to avoid interference from interatomic forces. The reaction can be represented as:
X(g) + energy → X⁺(g) + e⁻
Where:
- X(g) represents the neutral gaseous atom
- X⁺(g) represents the resulting gaseous cation
- e⁻ represents the removed electron
Importantly, ionization energy is always a positive value, signifying that energy is required to remove an electron. The stronger the attraction between the nucleus and the electron, the higher the ionization energy.
Factors Affecting Ionization Energy
Several factors influence the magnitude of ionization energy:
-
Nuclear Charge: A higher nuclear charge (more protons) leads to a stronger attraction for electrons, resulting in a higher ionization energy. The positive charge of the nucleus pulls the negatively charged electrons more strongly, making them harder to remove.
-
Atomic Radius: A larger atomic radius implies a greater distance between the nucleus and the valence electrons. The weaker electrostatic attraction between the nucleus and the electrons in larger atoms results in lower ionization energy. The electrons are further away and experience less pull from the nucleus.
-
Shielding Effect: Inner electrons shield the outer electrons from the full positive charge of the nucleus. This shielding effect reduces the effective nuclear charge experienced by the outermost electrons, thus lowering the ionization energy. More inner electrons mean more shielding, reducing the net attraction experienced by the valence electron.
-
Electron Configuration: A filled or half-filled subshell is more stable than a partially filled subshell. Removing an electron from a stable configuration requires more energy. This stability arises from electron-electron repulsions and exchange energies.
Successive Ionization Energies
It's possible to remove more than one electron from an atom. Each subsequent electron removal requires progressively more energy. This is known as successive ionization energy. The first ionization energy (IE₁) is always the lowest because it involves removing an electron from a neutral atom. Subsequent ionization energies (IE₂, IE₃, etc.) increase because the remaining electrons experience a greater effective nuclear charge as more electrons are removed. Large jumps in ionization energy often indicate the removal of an electron from a different principal energy level.
Electron Affinity: The Energy Change Upon Electron Gain
Electron affinity (EA) is the change in energy that occurs when an electron is added to a neutral gaseous atom to form a negative ion (anion). Unlike ionization energy, electron affinity can be either positive or negative.
-
Negative electron affinity: Indicates that energy is released when an electron is added. The atom readily accepts an electron, resulting in a more stable configuration. This means the anion is more stable than the neutral atom.
-
Positive electron affinity: Indicates that energy is required to add an electron. The atom is less likely to accept an electron. This happens when adding an electron to a stable electron configuration leads to increased electron-electron repulsion, making the process energetically unfavorable.
Factors Affecting Electron Affinity
Several factors affect the electron affinity of an atom:
-
Nuclear Charge: Similar to ionization energy, a higher nuclear charge generally leads to a more negative electron affinity (more energy released when an electron is added). The increased positive charge of the nucleus attracts the added electron more strongly.
-
Atomic Radius: A larger atomic radius results in a less negative (or even positive) electron affinity. The added electron is further away from the nucleus and experiences less attraction, making the process less energetically favorable.
-
Electron-Electron Repulsion: Adding an electron to an atom can increase electron-electron repulsion, particularly if the atom already has a lot of electrons. This repulsion makes it harder to add another electron and can lead to a positive electron affinity.
-
Electron Configuration: Adding an electron to a stable configuration (filled or half-filled subshell) will generally result in a less negative electron affinity or even a positive one. This is because adding to a stable state doesn't lead to a significant reduction in overall energy.
Trends in Ionization Energy and Electron Affinity Across the Periodic Table
Both ionization energy and electron affinity exhibit predictable trends across the periodic table:
-
Ionization Energy:
- Increases across a period: Across a period, the nuclear charge increases while the atomic radius remains relatively constant, leading to stronger attraction for electrons and higher ionization energy.
- Decreases down a group: Down a group, the atomic radius increases significantly, leading to weaker attraction for electrons and lower ionization energy. The increased shielding effect also contributes to this trend.
-
Electron Affinity:
- Generally increases across a period: Although there are exceptions, there is a general increase in the magnitude of negative electron affinity across a period due to increasing nuclear charge and relatively constant atomic radius.
- Generally decreases down a group: The increase in atomic radius down a group leads to a decrease in the magnitude of negative electron affinity.
Applications of Ionization Energy and Electron Affinity
Understanding ionization energy and electron affinity has various applications:
-
Predicting Chemical Reactivity: Atoms with low ionization energies are more likely to lose electrons and form cations, while those with high electron affinities are more likely to gain electrons and form anions. This helps predict the formation of ionic compounds.
-
Spectroscopy: Ionization energies can be determined experimentally using spectroscopic techniques like photoelectron spectroscopy. The energy required to remove an electron provides information about the electronic structure of the atom.
-
Materials Science: Ionization energies and electron affinities play a crucial role in understanding the behavior of materials, such as semiconductors. The ability to easily remove or add electrons determines the electrical conductivity of a material.
-
Analytical Chemistry: Techniques such as flame photometry and atomic absorption spectroscopy rely on the excitation and ionization of atoms for quantitative analysis.
-
Environmental Science: Understanding the ionization energies of atmospheric components helps model chemical reactions in the atmosphere, such as the ozone depletion cycle.
Conclusion
Ionization energy and electron affinity are fundamental concepts in chemistry that provide valuable insights into atomic structure and reactivity. Their trends across the periodic table, coupled with the understanding of the factors influencing them, allow for predictions about the chemical behavior of elements and compounds. These concepts are essential for many scientific disciplines, including materials science, environmental science, and analytical chemistry, showcasing their broad applicability and significance in scientific exploration. The interplay between nuclear charge, atomic radius, shielding, and electron configuration dictates these crucial atomic properties and provides a framework for understanding the diverse world of chemical reactions and bonding. Further exploration into these concepts reveals a more nuanced and complete picture of the atomic world.
Latest Posts
Latest Posts
-
Definition Of A Theory In Sociology
Mar 18, 2025
-
Is Mass A Chemical Or Physical Property
Mar 18, 2025
-
A Relationship Between Two Organisms In Which Both Organisms Benefit
Mar 18, 2025
-
How To Compute Cost Per Equivalent Unit
Mar 18, 2025
-
What Are The Physical Properties Of A Metal
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Understanding The Definitions Of Ionization Energy And Electron Affinity . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
