Understanding The Difference Between Strong And Weak Acids
Muz Play
Mar 24, 2025 · 6 min read
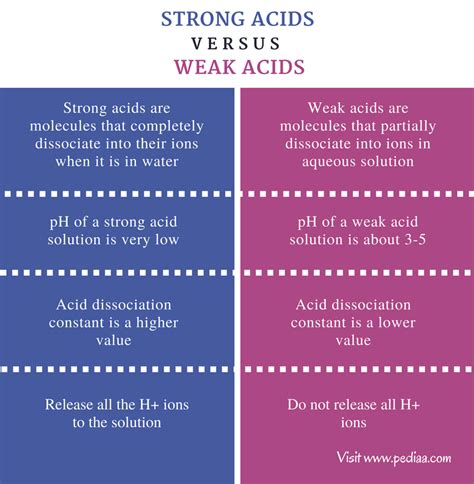
Table of Contents
Understanding the Difference Between Strong and Weak Acids
Acids are a fundamental concept in chemistry, playing crucial roles in numerous natural and industrial processes. Understanding the difference between strong and weak acids is essential for anyone studying chemistry, from high school students to advanced researchers. This comprehensive guide delves into the intricacies of acid strength, explaining the underlying principles, providing clear examples, and highlighting the practical implications of this distinction.
Defining Acids: A Brief Overview
Before diving into the differences, let's establish a common understanding of what constitutes an acid. In the simplest terms, an acid is a substance that donates a proton (H⁺ ion) to another substance, a process known as protonation. This definition is based on the Brønsted-Lowry acid-base theory, one of the most widely accepted models for understanding acid-base reactions. Another important theory, the Lewis theory, defines an acid as an electron-pair acceptor. While both definitions are valuable, the Brønsted-Lowry definition is more commonly used when discussing the strength of acids.
The Crucial Distinction: Strong vs. Weak Acids
The key difference between strong and weak acids lies in their degree of ionization in aqueous solutions (water).
Strong acids completely dissociate (ionize) into their constituent ions when dissolved in water. This means that every molecule of the strong acid donates its proton to a water molecule, forming hydronium ions (H₃O⁺) and the conjugate base of the acid. This process is essentially irreversible under normal conditions.
Weak acids, on the other hand, only partially dissociate in water. Only a small fraction of the weak acid molecules donate their protons, resulting in an equilibrium between the undissociated acid molecules and their ions. This equilibrium is described by an acid dissociation constant, Ka.
Understanding Acid Dissociation Constant (Ka)
The acid dissociation constant (Ka) is a quantitative measure of the strength of a weak acid. It represents the equilibrium constant for the dissociation reaction of the acid in water. A larger Ka value indicates a stronger weak acid, signifying a greater degree of dissociation. The Ka value is expressed as:
Ka = [H₃O⁺][A⁻] / [HA]
Where:
- [H₃O⁺] is the concentration of hydronium ions
- [A⁻] is the concentration of the conjugate base
- [HA] is the concentration of the undissociated weak acid
The pKa is often used instead of Ka because it's easier to handle numerically. pKa is the negative logarithm (base 10) of Ka:
pKa = -log₁₀(Ka)
A lower pKa value indicates a stronger weak acid.
Examples of Strong and Weak Acids
It's helpful to visualize the difference with concrete examples:
Common Strong Acids:
- Hydrochloric acid (HCl): Found in stomach acid and used in industrial processes. It completely dissociates into H⁺ and Cl⁻ ions in water.
- Sulfuric acid (H₂SO₄): A highly corrosive acid used extensively in industry, including fertilizer production and petroleum refining. It undergoes two dissociation steps, both of which are essentially complete in dilute solutions.
- Nitric acid (HNO₃): Used in the production of fertilizers and explosives. It completely dissociates into H⁺ and NO₃⁻ ions in water.
- Hydrobromic acid (HBr): A strong acid commonly used in laboratory settings.
- Hydroiodic acid (HI): Another strong acid with similar applications to HBr.
- Perchloric acid (HClO₄): One of the strongest acids known, requiring special handling due to its corrosive nature.
Common Weak Acids:
- Acetic acid (CH₃COOH): Found in vinegar, it only partially dissociates in water, resulting in a relatively low concentration of H⁺ ions.
- Carbonic acid (H₂CO₃): Present in carbonated drinks and blood, it plays a critical role in regulating blood pH. It's a diprotic acid, meaning it can donate two protons, but its dissociation is weak.
- Phosphoric acid (H₃PO₄): Used in fertilizers and food additives, it's a triprotic acid that undergoes three stages of dissociation, each with a progressively weaker tendency.
- Hydrofluoric acid (HF): While it's a weak acid, it's highly corrosive and dangerous. Its weak dissociation is due to the strong hydrogen-fluorine bond.
- Formic acid (HCOOH): Found in ant stings and some plants, it has a characteristic pungent odor.
- Benzoic acid (C₇H₆O₂): A weak organic acid used as a preservative.
Practical Implications of Acid Strength
The distinction between strong and weak acids has significant implications in various fields:
-
pH Calculation: The pH of a strong acid solution can be easily calculated from its concentration, as it completely dissociates. Calculating the pH of a weak acid solution requires the use of the Ka value and the equilibrium expression.
-
Buffer Solutions: Weak acids are essential components of buffer solutions, which resist changes in pH when small amounts of acid or base are added. The buffer capacity is directly related to the pKa of the weak acid and its conjugate base.
-
Titration Curves: Titration curves for strong acid-strong base titrations differ significantly from those for weak acid-strong base titrations. The equivalence point for a strong acid-strong base titration occurs at pH 7, while for a weak acid-strong base titration, it occurs at a pH above 7.
-
Industrial Applications: The choice of acid in industrial processes depends critically on its strength. Strong acids are typically used for reactions requiring complete protonation, whereas weak acids might be preferred in situations where milder conditions are needed. For example, strong acids are utilized in the manufacturing of fertilizers, while weaker organic acids are used in food preservation.
-
Biological Systems: Many biological processes rely on weak acids and their conjugate bases to maintain a stable pH within cells and tissues. These weak acid-base systems act as buffers, protecting against drastic pH fluctuations that could harm biological molecules and processes. For example, the carbonic acid-bicarbonate buffer system plays a vital role in regulating blood pH.
Factors Affecting Acid Strength
Several factors influence the strength of an acid:
-
Bond Strength: Acids with weaker bonds between the hydrogen atom and the rest of the molecule tend to be stronger acids because the hydrogen ion is more easily released.
-
Electronegativity: The electronegativity of the atom bonded to the hydrogen atom affects the polarity of the bond and the ease with which the hydrogen ion is released. Higher electronegativity leads to a stronger acid.
-
Size of the Conjugate Base: Larger conjugate bases are generally more stable, making it easier for the acid to donate a proton.
-
Resonance Stabilization: If the conjugate base is stabilized through resonance, the acid will be stronger. Resonance delocalizes the negative charge over multiple atoms, increasing stability.
Conclusion
The difference between strong and weak acids is a fundamental concept in chemistry with far-reaching implications. Understanding the principles of acid dissociation, the significance of the Ka and pKa values, and the various factors affecting acid strength is crucial for comprehending a vast array of chemical and biological processes. This knowledge is essential not only for academic pursuits but also for practical applications in numerous industries and fields, reinforcing the importance of mastering this key concept. By appreciating the nuanced distinctions between strong and weak acids, one can better grasp the intricate workings of the chemical world and apply this knowledge to solve real-world problems.
Latest Posts
Latest Posts
-
Why Are Tertiary Carbocations More Stable
Mar 26, 2025
-
How To Draw An Integral Sign
Mar 26, 2025
-
Which Plane Divides The Body Into Superior And Inferior Sections
Mar 26, 2025
-
Difference Between Substrate Level Phosphorylation And Oxidative Phosphorylation
Mar 26, 2025
-
5 Steps Of The Listening Process
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Understanding The Difference Between Strong And Weak Acids . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
