Using First And Second Order Integrated Rate Laws
Muz Play
Mar 20, 2025 · 6 min read
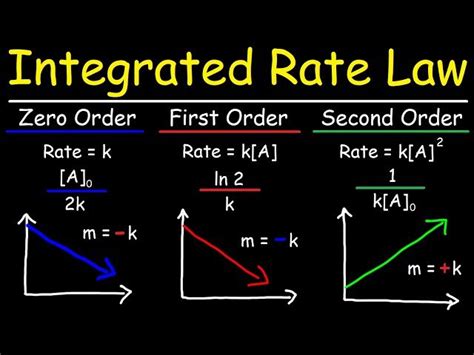
Table of Contents
Using First and Second Order Integrated Rate Laws: A Comprehensive Guide
Chemical kinetics is a fundamental aspect of chemistry, dealing with the rates of chemical reactions. Understanding these rates is crucial for predicting reaction outcomes, optimizing industrial processes, and developing new catalytic systems. A cornerstone of chemical kinetics is the concept of integrated rate laws, which provide a mathematical description of how reactant concentrations change over time. This article dives deep into the application and understanding of first and second-order integrated rate laws.
Understanding Rate Laws
Before delving into integrated rate laws, let's establish a foundational understanding of rate laws themselves. The rate law expresses the relationship between the rate of a reaction and the concentrations of the reactants. For a general reaction:
aA + bB → cC + dD
The rate law is typically expressed as:
Rate = k[A]<sup>m</sup>[B]<sup>n</sup>
where:
- Rate: The rate of the reaction (often expressed as change in concentration per unit time).
- k: The rate constant, a temperature-dependent proportionality constant.
- [A] and [B]: The concentrations of reactants A and B.
- m and n: The reaction orders with respect to A and B, respectively. These are experimentally determined and are not necessarily equal to the stoichiometric coefficients (a and b).
The overall reaction order is the sum of the individual orders (m + n).
First-Order Integrated Rate Law
A first-order reaction is one where the rate is directly proportional to the concentration of a single reactant. For the reaction:
A → products
The rate law is:
Rate = k[A]
Integrating this rate law with respect to time yields the first-order integrated rate law:
ln[A]<sub>t</sub> - ln[A]<sub>0</sub> = -kt
or equivalently:
ln[A]<sub>t</sub> = -kt + ln[A]<sub>0</sub>
where:
- [A]<sub>t</sub>: Concentration of A at time t.
- [A]<sub>0</sub>: Initial concentration of A (at time t = 0).
- k: Rate constant.
- t: Time.
This equation has the form of a straight line (y = mx + c), where ln[A]<sub>t</sub> is the y-axis, t is the x-axis, -k is the slope, and ln[A]<sub>0</sub> is the y-intercept. This linearity allows for easy determination of the rate constant (k) from experimental data by plotting ln[A]<sub>t</sub> versus time.
Applications of the First-Order Integrated Rate Law
The first-order integrated rate law has numerous applications across various fields:
-
Radioactive decay: The decay of radioactive isotopes follows first-order kinetics. The half-life (t<sub>1/2</sub>), the time it takes for half of the reactant to decay, is a constant for first-order reactions and can be calculated using the equation: t<sub>1/2</sub> = ln(2)/k
-
Pharmacokinetics: The elimination of drugs from the body often follows first-order kinetics. This allows for the prediction of drug concentrations in the bloodstream over time.
-
Atmospheric chemistry: The decomposition of certain atmospheric pollutants follows first-order kinetics. This helps in understanding and modeling air pollution dynamics.
-
Enzyme kinetics (at low substrate concentration): At low substrate concentrations, enzyme-catalyzed reactions can exhibit first-order kinetics.
Second-Order Integrated Rate Laws
A second-order reaction can involve either a single reactant with a second-order dependence or two reactants each with a first-order dependence.
Case 1: Second-Order Reaction with a Single Reactant
For the reaction:
2A → products
The rate law is:
Rate = k[A]<sup>2</sup>
Integrating this rate law gives the second-order integrated rate law:
1/[A]<sub>t</sub> - 1/[A]<sub>0</sub> = kt
Similar to the first-order case, this equation is linear when 1/[A]<sub>t</sub> is plotted against time. The slope of the line gives the rate constant k. The half-life for a second-order reaction with a single reactant is dependent on the initial concentration: t<sub>1/2</sub> = 1/(k[A]<sub>0</sub>)
Case 2: Second-Order Reaction with Two Reactants
Consider the reaction:
A + B → products
If the reaction is first-order with respect to both A and B, the rate law is:
Rate = k[A][B]
Integrating this rate law is more complex and usually requires specific knowledge of the initial concentrations and their relative amounts. Simplified forms exist when the initial concentrations of A and B are equal or one is significantly larger than the other (pseudo-first-order conditions).
Pseudo-First-Order Kinetics
If one reactant is present in significantly large excess compared to the other, the concentration of the reactant in excess remains essentially constant during the reaction. This simplifies the rate law. For instance, in the A + B reaction, if [B] >> [A], then [B] is approximately constant throughout the reaction, making the rate law appear first-order:
Rate ≈ k'[A]
where k' = k[B] This is called pseudo-first-order kinetics. The observed rate constant (k') depends on the concentration of the reactant in excess. This simplification is commonly used to simplify experimental analysis.
Applications of Second-Order Integrated Rate Laws
Second-order reactions are prevalent in various chemical processes:
-
Gas-phase reactions: Many gas-phase reactions, particularly those involving bimolecular collisions, follow second-order kinetics.
-
Enzyme kinetics (at high substrate concentration): At high substrate concentrations, enzyme-catalyzed reactions can become second-order, with the rate dependent on both enzyme and substrate concentrations.
-
Nucleophilic substitution reactions (SN2): These organic reactions often follow second-order kinetics.
Distinguishing Between Reaction Orders
It's crucial to experimentally determine the order of a reaction. This typically involves measuring reactant concentrations at different times and analyzing the data using the integrated rate laws. Plotting the appropriate function of concentration versus time will yield a straight line only for the correct reaction order.
- First-order: A plot of ln[A]<sub>t</sub> versus time will be linear.
- Second-order (single reactant): A plot of 1/[A]<sub>t</sub> versus time will be linear.
Analyzing the half-life can also provide clues. A constant half-life indicates a first-order reaction, while a half-life that is inversely proportional to the initial concentration points toward a second-order reaction (single reactant).
Advanced Considerations
The discussion above focuses primarily on simple reaction mechanisms. Real-world reactions can be far more complex, involving multiple steps, intermediate species, and reversible reactions. Techniques such as the method of initial rates, relaxation methods, and more advanced mathematical treatments are required to analyze these intricate systems. Furthermore, the temperature dependence of the rate constant (k) is described by the Arrhenius equation, providing valuable insight into the activation energy of the reaction.
Conclusion
Understanding and applying first and second-order integrated rate laws is essential for analyzing chemical reaction kinetics. By mastering these concepts and their corresponding mathematical tools, chemists and engineers can predict reaction rates, optimize processes, design new catalysts and gain a fundamental understanding of the mechanisms that govern chemical change. The ability to distinguish between reaction orders through experimental analysis and applying the appropriate integrated rate law is crucial for accurate data interpretation and predictive modeling in numerous scientific and engineering fields. Remember that while this guide provides a strong foundation, exploring more advanced topics will further enhance your understanding and ability to tackle more intricate kinetic problems.
Latest Posts
Latest Posts
-
Concentration Of A Sodium Chloride Solution
Mar 20, 2025
-
Titration Acid And Base Lab Report
Mar 20, 2025
-
Do Protists Have A Cell Wall
Mar 20, 2025
-
All Matter Can Be Classified As
Mar 20, 2025
-
Measurement Of The Amount Of Matter In An Object
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Using First And Second Order Integrated Rate Laws . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
