All Matter Can Be Classified As
Muz Play
Mar 20, 2025 · 7 min read
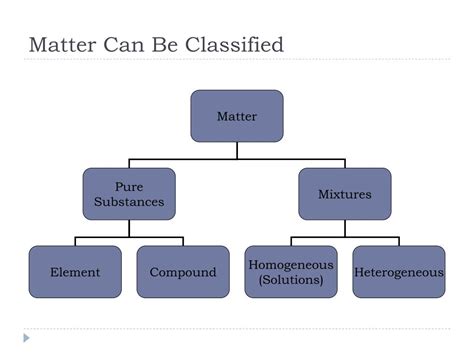
Table of Contents
All Matter Can Be Classified As: A Comprehensive Guide to the States and Properties of Matter
The universe, in all its vastness and complexity, is fundamentally composed of matter. But what is matter? And how can we effectively classify the myriad forms it takes? This comprehensive guide dives deep into the classification of matter, exploring its different states, properties, and the underlying principles that govern its behavior. We'll journey from the macroscopic world of everyday objects to the microscopic realm of atoms and molecules, unraveling the fascinating intricacies of the material world.
The Fundamental Classification: Pure Substances and Mixtures
At the most basic level, all matter can be classified into two broad categories: pure substances and mixtures. This distinction hinges on the uniformity of composition and the ability to separate components.
Pure Substances: Uniformity at the Molecular Level
Pure substances have a fixed chemical composition and uniform properties throughout. This means that no matter where you sample a pure substance from, its chemical makeup and physical properties will remain consistent. Pure substances can be further categorized into:
Elements: The Building Blocks
Elements are the simplest form of pure substances. They consist of only one type of atom. These atoms cannot be broken down into simpler substances through chemical means. The periodic table organizes all known elements, highlighting their properties and relationships. Examples include oxygen (O), gold (Au), and hydrogen (H).
Key Characteristics of Elements:
- Unique atomic number: Defined by the number of protons in the nucleus.
- Characteristic properties: Each element exhibits a unique set of physical and chemical properties.
- Indivisible by chemical means: Elements cannot be broken down further using chemical reactions.
Compounds: Elements United
Compounds are pure substances formed when two or more different elements chemically combine in a fixed ratio. This combination results in a new substance with properties distinct from its constituent elements. For instance, water (H₂O) is a compound formed from hydrogen and oxygen, with dramatically different properties than either element alone.
Key Characteristics of Compounds:
- Fixed composition: The ratio of elements in a compound is always constant.
- New properties: Compounds have properties different from their constituent elements.
- Separable by chemical means: Compounds can be broken down into their constituent elements through chemical reactions.
Mixtures: A Blend of Substances
Unlike pure substances, mixtures are composed of two or more substances physically combined. These substances retain their individual properties and can be separated by physical methods. Mixtures are further divided into:
Homogeneous Mixtures: Uniformity Throughout
Homogeneous mixtures have a uniform composition throughout. This means the components are evenly distributed, and a sample taken from any part of the mixture will have the same composition. Examples include saltwater, air, and sugar dissolved in water.
Key Characteristics of Homogeneous Mixtures:
- Uniform composition: Components are evenly distributed.
- Invisible components: Individual components are not visibly distinguishable.
- Easily separable: Components can be separated using physical methods like distillation or filtration.
Heterogeneous Mixtures: Non-Uniform Composition
Heterogeneous mixtures have a non-uniform composition. This means the components are not evenly distributed, and different parts of the mixture will have different compositions. Examples include sand and water, oil and water, and a salad.
Key Characteristics of Heterogeneous Mixtures:
- Non-uniform composition: Components are unevenly distributed.
- Visible components: Individual components are visibly distinguishable.
- Separable: Components can be separated using physical methods like decantation or filtration.
The States of Matter: Solid, Liquid, and Gas
Beyond the fundamental classification of pure substances and mixtures, matter also exists in different physical states, primarily: solid, liquid, and gas. The state of matter is determined by the arrangement and interactions of its constituent particles (atoms, molecules, or ions).
Solids: Order and Structure
Solids have a fixed shape and volume. Their particles are closely packed together in a highly ordered arrangement, resulting in strong intermolecular forces. This strong attraction restricts the movement of particles, leading to rigidity and a definite shape. Solids can be further classified as crystalline (ordered arrangement) or amorphous (disordered arrangement).
Key Characteristics of Solids:
- Fixed shape and volume: Maintains its shape and volume regardless of container.
- Incompressible: Particles are closely packed, making compression difficult.
- High density: Particles are closely packed, resulting in high density.
Liquids: Flow and Adaptability
Liquids have a fixed volume but a variable shape. Their particles are closely packed but can move past each other, leading to fluidity and the ability to adapt to the shape of their container. The intermolecular forces in liquids are weaker than in solids, allowing for more movement.
Key Characteristics of Liquids:
- Fixed volume, variable shape: Adapts to the shape of its container while maintaining its volume.
- Slightly compressible: Particles have some space between them, allowing slight compression.
- High density (generally): Particles are relatively close, resulting in high density.
Gases: Freedom and Expansion
Gases have neither a fixed shape nor a fixed volume. Their particles are widely spaced and move randomly at high speeds. The weak intermolecular forces allow gases to expand to fill their containers completely.
Key Characteristics of Gases:
- Variable shape and volume: Expands to fill its container.
- Highly compressible: Large spaces between particles allow for easy compression.
- Low density: Particles are widely spaced, resulting in low density.
Beyond the Three Main States: Plasma and Bose-Einstein Condensates
While solid, liquid, and gas are the most commonly encountered states of matter, other states exist under specific conditions:
Plasma: Ionized Gas
Plasma is an ionized gas composed of free-moving ions and electrons. It's formed when sufficient energy is added to a gas, causing its atoms to lose electrons. Plasma is the most abundant state of matter in the universe, comprising stars, nebulae, and the Sun.
Key Characteristics of Plasma:
- Ionized gas: Contains free-moving ions and electrons.
- High energy: Requires significant energy input to form.
- Conducts electricity: Free-moving charged particles facilitate electrical conductivity.
Bose-Einstein Condensates: Quantum Matter
Bose-Einstein condensates (BECs) are a state of matter formed at extremely low temperatures, close to absolute zero (-273.15°C). At these temperatures, a large fraction of bosons occupy the lowest quantum state, resulting in a collective state with macroscopic quantum phenomena. This state exhibits unique properties, including superfluidity and coherence.
Key Characteristics of Bose-Einstein Condensates:
- Ultra-low temperature: Formed near absolute zero.
- Macroscopic quantum phenomena: Exhibits collective quantum behavior.
- Superfluidity: Flows without viscosity.
Properties of Matter: Physical and Chemical
Understanding the classification of matter is incomplete without examining its properties. These properties can be broadly categorized as physical and chemical:
Physical Properties: Observable Characteristics
Physical properties are characteristics that can be observed or measured without changing the chemical composition of the substance. Examples include color, density, melting point, boiling point, and conductivity.
Examples of Physical Properties:
- Color: The visual appearance of a substance.
- Density: Mass per unit volume.
- Melting point: The temperature at which a solid changes to a liquid.
- Boiling point: The temperature at which a liquid changes to a gas.
- Conductivity: The ability to conduct heat or electricity.
Chemical Properties: Reactivity and Transformation
Chemical properties describe how a substance reacts with other substances or transforms into other substances. These properties are observed only when a chemical change occurs, altering the chemical composition. Examples include flammability, reactivity with acids, and oxidation.
Examples of Chemical Properties:
- Flammability: The ability to burn in the presence of oxygen.
- Reactivity with acids: How a substance reacts when exposed to acids.
- Oxidation: The reaction of a substance with oxygen.
Conclusion: A Diverse and Dynamic Material World
The classification of matter is a cornerstone of chemistry and physics. From the simplest elements to complex mixtures and the diverse states in which matter exists, understanding these classifications provides a framework for comprehending the physical world around us. By exploring the properties and behaviors of matter, we can unlock insights into the universe's fundamental building blocks and the intricate processes that shape its form. This journey into the nature of matter is ongoing, with continued research revealing new insights into its remarkable complexity and diversity. The ongoing exploration of exotic states of matter, like BECs, highlights the boundless possibilities and ever-evolving understanding of the material world.
Latest Posts
Latest Posts
-
How To Create A Wet Mount Slide
Mar 21, 2025
-
Person In Environment Perspective Social Work
Mar 21, 2025
-
Calculating The Ph At The Equivalence Point
Mar 21, 2025
-
How Does Temperature Affect Diffusion Rate
Mar 21, 2025
-
What Are The Units Of Wavelength
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about All Matter Can Be Classified As . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
