How Does Temperature Affect Diffusion Rate
Muz Play
Mar 21, 2025 · 6 min read
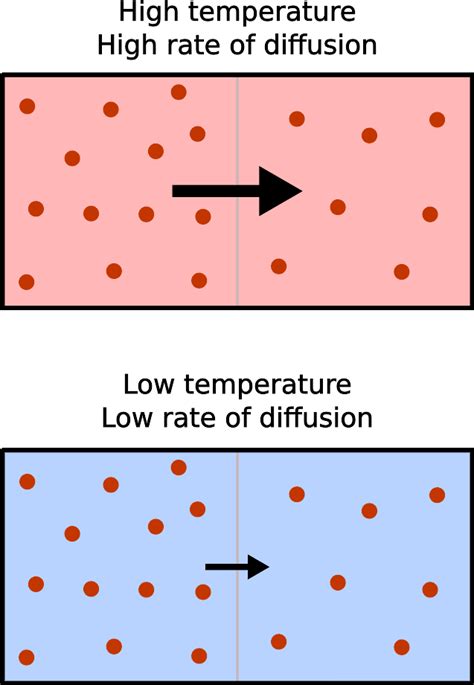
Table of Contents
How Does Temperature Affect Diffusion Rate? A Comprehensive Guide
Diffusion, the net movement of particles from a region of higher concentration to a region of lower concentration, is a fundamental process in many natural phenomena and technological applications. Understanding how various factors influence diffusion rates is crucial in numerous fields, from biology and chemistry to material science and engineering. This article will delve deep into the relationship between temperature and diffusion rate, exploring the underlying mechanisms and providing real-world examples.
The Kinetic Theory and Diffusion
To understand the temperature-diffusion relationship, we must first grasp the basics of the kinetic theory of matter. This theory postulates that matter is composed of constantly moving particles (atoms, molecules, or ions). The kinetic energy of these particles is directly proportional to the absolute temperature (Kelvin). This means that as temperature increases, the particles move faster and more vigorously.
Higher Temperature, Higher Kinetic Energy
This increased kinetic energy is the key to understanding the effect of temperature on diffusion. At higher temperatures, particles possess greater kinetic energy, leading to more frequent and forceful collisions. These collisions, in turn, facilitate the movement of particles from areas of high concentration to areas of low concentration, thereby accelerating the diffusion process.
Increased Collision Frequency and Diffusion Rate
The increased frequency and intensity of collisions at higher temperatures don't just randomly scatter the particles. Instead, they propel them further and faster, leading to a significantly higher rate of diffusion. This isn't just about individual particles moving faster; it's about the overall net movement of particles from high to low concentration being accelerated.
The Mathematical Relationship: Fick's First Law
The quantitative relationship between diffusion rate and temperature is often described by Fick's First Law of Diffusion. While the law itself doesn't explicitly include temperature, the diffusion coefficient (D), a crucial parameter in Fick's Law, is highly temperature-dependent.
Fick's First Law: J = -D (dc/dx)
- J: Diffusion flux (amount of substance diffusing per unit area per unit time)
- D: Diffusion coefficient (a measure of how readily a substance diffuses)
- dc/dx: Concentration gradient (change in concentration over distance)
The negative sign indicates that diffusion occurs down the concentration gradient, from high to low concentration. The diffusion coefficient, D, is the key to understanding the temperature dependence.
Temperature Dependence of the Diffusion Coefficient (D)
The diffusion coefficient (D) is not a constant; it varies significantly with temperature. The relationship is often approximated by the Arrhenius equation:
Arrhenius Equation: D = D₀ exp(-Ea/RT)
- D: Diffusion coefficient
- D₀: Pre-exponential factor (a constant related to the vibrational frequency of atoms)
- Ea: Activation energy (energy barrier that particles must overcome to diffuse)
- R: Ideal gas constant
- T: Absolute temperature (in Kelvin)
This equation reveals an exponential relationship between the diffusion coefficient and temperature. As temperature (T) increases, the exponential term (-Ea/RT) decreases, leading to a significant increase in the diffusion coefficient (D). This explains why diffusion rates increase so dramatically with temperature.
The Activation Energy (Ea) and its Role
The activation energy (Ea) represents the energy barrier that particles must overcome to move from one location to another. It's essentially the energy required to break intermolecular or interatomic bonds and create a pathway for diffusion. Substances with lower activation energies will show a greater increase in diffusion rate with temperature compared to those with higher activation energies.
Real-World Examples of Temperature's Effect on Diffusion
The influence of temperature on diffusion is observable in numerous real-world scenarios:
1. Cooking:
Think about cooking a steak. The heat speeds up the diffusion of heat within the meat, ensuring it cooks evenly. Similarly, the diffusion of flavors and aromas within the food is also significantly faster at higher temperatures. That’s why you want to let your stew simmer long enough – it needs time for those tasty flavors to diffuse throughout.
2. Smelling Perfume:
The scent of perfume diffuses more rapidly in warm air than in cold air. This is because the fragrance molecules move faster at higher temperatures, spreading out more quickly and allowing you to smell the perfume more intensely.
3. Melting of Solids:
The melting of a solid is a diffusion process, where particles break free from their fixed positions and diffuse throughout the liquid phase. This transition happens much faster at higher temperatures, because the increased kinetic energy helps overcome the intermolecular forces holding the solid together.
4. Gas Diffusion:
The rate at which gases mix is significantly influenced by temperature. A balloon filled with helium will deflate faster in a warm room than in a cold room due to the faster diffusion of helium molecules through the balloon material at higher temperatures.
5. Biological Systems:
Enzyme activity, a critical process in biological systems, is highly temperature-dependent. Enzymes facilitate diffusion-based reactions, and their effectiveness is optimized within a specific temperature range. Extreme temperatures can denature enzymes, disrupting their function and hindering diffusion-dependent processes.
6. Metallurgy:
In metallurgy, the diffusion of atoms in metal alloys is essential for various heat treatments. Higher temperatures accelerate diffusion processes like annealing (stress relief), which is critical for improving the strength and ductility of materials.
7. Semiconductor Manufacturing:
The manufacturing of semiconductor devices relies heavily on diffusion processes to control the concentration of dopants in silicon wafers. The diffusion temperature significantly influences the depth and concentration profile of the dopants.
Factors Beyond Temperature Affecting Diffusion Rate
While temperature is a dominant factor, it's crucial to remember that other factors also influence diffusion rates:
- Concentration Gradient: A steeper concentration gradient leads to a faster diffusion rate.
- Diffusion Medium: The nature of the medium (solid, liquid, gas) affects the diffusion coefficient. Diffusion is generally faster in gases than in liquids, and faster in liquids than in solids.
- Particle Size and Shape: Larger and more irregularly shaped particles generally diffuse slower than smaller, spherical particles.
- Pressure (for gases): Higher pressure in gases leads to increased collision frequency, thereby influencing diffusion.
Conclusion
The relationship between temperature and diffusion rate is a powerful and fundamental principle with broad implications across numerous scientific and technological disciplines. The exponential increase in diffusion rate with temperature, as described by the Arrhenius equation, highlights the crucial role of kinetic energy in driving this essential process. Understanding this relationship is key to controlling and optimizing numerous applications, from cooking to the fabrication of advanced materials. By carefully controlling temperature, we can fine-tune diffusion rates to achieve desired outcomes in a wide array of fields. Further research into the intricacies of diffusion, including the effects of multiple factors, will continue to yield advancements in various fields of study.
Latest Posts
Latest Posts
-
What Does Newtons First Law Say
Mar 28, 2025
-
Does Ionic Have High Boiling Point
Mar 28, 2025
-
Is Dipole Dipole Polar Or Nonpolar
Mar 28, 2025
-
A Hydrocarbon Is Any Compound That Contains
Mar 28, 2025
-
In Order To Break A Bond Energy Must Be
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about How Does Temperature Affect Diffusion Rate . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
