Calculating The Ph At The Equivalence Point
Muz Play
Mar 21, 2025 · 5 min read
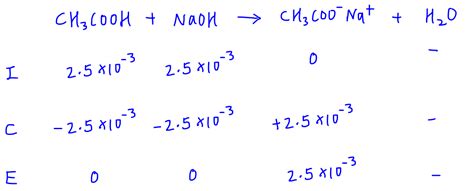
Table of Contents
Calculating the pH at the Equivalence Point: A Comprehensive Guide
Determining the pH at the equivalence point of a titration is crucial for understanding the reaction's stoichiometry and selecting appropriate indicators. This point signifies the complete neutralization of the analyte by the titrant, offering valuable insights into the solution's properties. This article delves into the intricacies of calculating the pH at the equivalence point for various titrations, including strong acid-strong base, weak acid-strong base, weak base-strong acid, and polyprotic acid-strong base titrations. We’ll explore the underlying principles and provide practical examples to solidify your understanding.
Understanding the Equivalence Point
The equivalence point in a titration marks the stoichiometric point where the moles of titrant added equal the moles of analyte present. It's a theoretical point, often visually approximated by the endpoint observed with an indicator. The pH at the equivalence point varies considerably depending on the strengths of the acid and base involved.
Strong Acid-Strong Base Titrations
Strong acid-strong base titrations are the simplest to analyze. Both the acid and base completely dissociate in solution. At the equivalence point, the solution contains only the salt formed from the neutralization reaction and water. Since the salt of a strong acid and a strong base is neutral, the pH at the equivalence point is 7.
Example: Titration of 100 mL of 0.1 M HCl (strong acid) with 0.1 M NaOH (strong base).
At the equivalence point, the moles of HCl = moles of NaOH. The volume of NaOH required is 100 mL. The resulting solution contains only NaCl and water. Therefore, the pH = 7.
Weak Acid-Strong Base Titrations
Titrating a weak acid with a strong base presents a more complex scenario. At the equivalence point, the solution contains the conjugate base of the weak acid. This conjugate base undergoes hydrolysis, reacting with water to produce hydroxide ions, resulting in a pH greater than 7.
To calculate the pH:
-
Determine the concentration of the conjugate base: This involves considering the dilution that occurs during the titration.
-
Calculate the Kb of the conjugate base: Kb = Kw/Ka, where Kw is the ion product of water (1.0 x 10⁻¹⁴ at 25°C) and Ka is the acid dissociation constant of the weak acid.
-
Use an ICE table (Initial, Change, Equilibrium) to determine the hydroxide ion concentration: The ICE table helps track the changes in concentration during the hydrolysis reaction.
-
Calculate the pOH: pOH = -log[OH⁻]
-
Calculate the pH: pH = 14 - pOH
Example: Titration of 50 mL of 0.1 M acetic acid (Ka = 1.8 x 10⁻⁵) with 0.1 M NaOH.
At the equivalence point, 50 mL of NaOH is added. The concentration of acetate ion (conjugate base) is 0.05 M (due to dilution). Using the ICE table and Kb calculation, the pH can be determined to be greater than 7.
Weak Base-Strong Acid Titrations
Titrating a weak base with a strong acid is analogous to the weak acid-strong base titration, but in reverse. At the equivalence point, the solution contains the conjugate acid of the weak base, which undergoes hydrolysis to produce hydronium ions, leading to a pH less than 7.
The calculation process is similar to the weak acid-strong base titration, but instead of using Kb, we use Ka for the conjugate acid, Ka = Kw/Kb. The calculation involves an ICE table and determining the hydronium ion concentration to calculate the pH.
Example: Titration of 100 mL of 0.1 M ammonia (Kb = 1.8 x 10⁻⁵) with 0.1 M HCl.
At the equivalence point, the ammonium ion concentration is calculated, and its Ka value is used in an ICE table to determine the hydronium ion concentration and subsequently, the pH. The pH will be less than 7.
Polyprotic Acid-Strong Base Titrations
Polyprotic acids have multiple ionizable protons. Their titrations exhibit multiple equivalence points, each corresponding to the neutralization of a single proton. Calculating the pH at each equivalence point requires a stepwise approach, considering the successive dissociation constants (Ka1, Ka2, etc.).
The pH at each equivalence point is influenced by the equilibrium of the conjugate base formed at that stage. The calculation involves successive ICE tables and consideration of the relevant equilibrium constants.
Example: Titration of a diprotic acid like oxalic acid (H₂C₂O₄) with NaOH.
This titration will have two equivalence points. At the first equivalence point, the solution primarily contains HC₂O₄⁻, and its equilibrium with water determines the pH. At the second equivalence point, the solution contains C₂O₄²⁻, and its equilibrium determines the pH. The calculations are more complex and involve multiple equilibrium considerations.
Factors Affecting pH at the Equivalence Point
Several factors can influence the pH at the equivalence point, including:
- Temperature: The Kw value changes with temperature, affecting the pH calculations.
- Ionic strength: The presence of ions in the solution can influence the activity coefficients of the species involved, subtly affecting the pH.
- Concentration of the acid and base: While the stoichiometry is constant at the equivalence point, the concentration influences the absolute amounts of conjugate acid/base present, influencing the degree of hydrolysis and therefore the pH.
Choosing the Right Indicator
The appropriate indicator for a titration is chosen based on the pH at the equivalence point and the pH range over which the indicator changes color (its transition range). Indicators are weak acids or bases that exhibit different colors in their protonated and deprotonated forms. The indicator’s transition range should ideally encompass the equivalence point to ensure accurate determination.
Conclusion
Calculating the pH at the equivalence point requires a thorough understanding of acid-base chemistry and equilibrium principles. While simple for strong acid-strong base titrations, the calculations become more complex for weak acid-strong base, weak base-strong acid, and polyprotic acid titrations. Mastering these calculations is fundamental for accurate quantitative analysis and understanding the behavior of acid-base systems. Remember to always carefully consider the specific acid and base involved, their concentrations, and the relevant equilibrium constants (Ka and Kb) when performing these calculations. Accurate pH determination allows for precise endpoint determination during titrations, aiding in the precise quantitative analysis of unknown solutions. The principles discussed here are invaluable in various scientific fields, including chemistry, environmental science, and biochemistry.
Latest Posts
Latest Posts
-
Is Dipole Dipole Polar Or Nonpolar
Mar 28, 2025
-
A Hydrocarbon Is Any Compound That Contains
Mar 28, 2025
-
In Order To Break A Bond Energy Must Be
Mar 28, 2025
-
How Do You Divide A Square Root
Mar 28, 2025
-
Proof Of Derivative Of Inverse Trig Functions
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Calculating The Ph At The Equivalence Point . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
