Valid Digits In A Measurement Are Called
Muz Play
Mar 21, 2025 · 7 min read
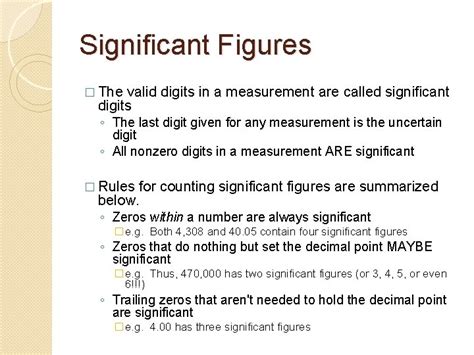
Table of Contents
Valid Digits in a Measurement: A Deep Dive into Significant Figures
Understanding significant figures is crucial for anyone working with measurements, whether in science, engineering, or everyday life. The accuracy of our calculations and the reliability of our conclusions depend heavily on correctly identifying and manipulating significant figures. So, what exactly are valid digits in a measurement? They are commonly referred to as significant figures. This comprehensive guide will delve into the intricacies of significant figures, explaining their meaning, how to identify them, and how to use them correctly in calculations.
What are Significant Figures?
Significant figures (also known as significant digits) are the digits in a number that carry meaning contributing to its measurement resolution. They represent the precision of a measurement. In essence, they tell us how much we can reliably trust the value of a measurement. A number with more significant figures indicates a more precise measurement than a number with fewer significant figures. Understanding this is critical because it directly impacts the accuracy of calculations and interpretations derived from those measurements.
Why are Significant Figures Important?
The importance of significant figures stems from the reality that measurements are never perfectly precise. Every measuring instrument has limitations, and there will always be some degree of uncertainty in the measurement. Significant figures help us communicate this uncertainty and avoid misrepresenting the precision of our results. Using the correct number of significant figures prevents us from implying a level of accuracy that doesn't exist. For example, claiming a measurement is 12.345 cm when your instrument only measures to the nearest tenth of a centimeter is misleading.
Identifying Significant Figures: The Rules
Identifying significant figures can be straightforward, but there are specific rules to follow:
Rule 1: Non-zero Digits are Always Significant
This is the most basic rule. Any digit that is not zero is always considered significant. For example, in the number 234, there are three significant figures (2, 3, and 4).
Rule 2: Zeros Between Non-zero Digits are Significant
Zeros placed between non-zero digits are significant. For example, in the number 1005, there are four significant figures (1, 0, 0, and 5). The zeros are significant because they are holding a place value between the 1 and the 5.
Rule 3: Leading Zeros are Never Significant
Leading zeros are zeros that appear before the first non-zero digit. These are placeholders and do not contribute to the precision of the measurement. For example, in the number 0.0045, there are only two significant figures (4 and 5). The leading zeros are merely indicating the decimal place.
Rule 4: Trailing Zeros in a Number Containing a Decimal Point are Significant
Trailing zeros are zeros that appear at the end of a number. If a number contains a decimal point, trailing zeros are significant. For example, in the number 12.00, there are four significant figures (1, 2, 0, and 0). The trailing zeros indicate the measurement's precision to the hundredths place.
Rule 5: Trailing Zeros in a Number Without a Decimal Point are Ambiguous
This is where it gets slightly trickier. Trailing zeros in a number without an explicitly written decimal point are ambiguous. For example, the number 1200 could have two, three, or four significant figures. To clarify the number of significant figures, scientific notation is recommended. We will explore scientific notation in more detail later.
Examples of Identifying Significant Figures
Let's look at some examples to solidify our understanding:
- 123.45: Five significant figures
- 0.0056: Two significant figures
- 100.0: Four significant figures (the trailing zeros after the decimal are significant)
- 1000: Ambiguous; could be one, two, three, or four significant figures
- 1.000 x 10³: Four significant figures (scientific notation removes the ambiguity)
- 2005: Four significant figures
- 0.002050: Four significant figures (note that the zero between 2 and 5 and the trailing zero are significant)
Significant Figures in Calculations
When performing calculations with measurements, the number of significant figures in the result must be handled carefully to reflect the uncertainty in the original measurements.
Addition and Subtraction
In addition and subtraction, the result should have the same number of decimal places as the measurement with the fewest decimal places.
Example:
12.34 + 5.6 + 1.234 = 19.174 Rounding to one decimal place (matching the least precise measurement, 5.6) gives us 19.2.
Multiplication and Division
In multiplication and division, the result should have the same number of significant figures as the measurement with the fewest significant figures.
Example:
12.34 x 5.6 = 69.104 Rounding to two significant figures (matching the least precise measurement, 5.6) gives us 69.
Scientific Notation and Significant Figures
Scientific notation is a valuable tool for expressing very large or very small numbers while clearly indicating the number of significant figures. It also removes the ambiguity associated with trailing zeros. A number expressed in scientific notation is written in the form: A x 10<sup>B</sup>, where A is a number between 1 and 10, and B is an integer exponent. Only the digits in A are considered significant.
Example:
The number 12000 written with three significant figures is 1.20 x 10<sup>4</sup>. The number of significant figures is unambiguous in this representation.
Rounding and Significant Figures
Rounding is essential to ensure that the final answer contains the correct number of significant figures. The general rule is to round up if the digit to be dropped is 5 or greater and round down if it is less than 5. However, if the digit to be dropped is exactly 5, and it is followed by zeros or nothing, round to the nearest even number. This helps to minimize bias in repeated rounding.
The Importance of Precision in Measurements
The concept of significant figures underlines the critical importance of precision in scientific measurements. Without careful consideration of significant figures, experimental errors could be magnified, leading to incorrect conclusions. For example, in a complex experiment involving multiple measurements, errors in significant figure handling could accumulate and drastically affect the final result.
Example Scenario:
Imagine a chemist calculating the yield of a chemical reaction. Each step of the experiment involves measurements with varying degrees of precision. If the chemist doesn't properly account for significant figures throughout the calculation, the final yield calculation will reflect an inaccurate precision, potentially leading to erroneous conclusions about the reaction's efficiency. This could have significant implications if the results are used to scale up the reaction for industrial purposes.
Real-World Applications of Significant Figures
Understanding significant figures extends beyond the scientific laboratory. It plays a vital role in various real-world applications:
-
Engineering: In civil, mechanical, or electrical engineering, precise measurements are paramount. Incorrect use of significant figures can lead to design flaws or safety concerns in construction, machine building, or electrical systems.
-
Manufacturing: In industrial production, the precision of manufacturing processes depends on accurate measurements. The correct application of significant figures ensures consistent product quality and prevents costly errors.
-
Medical Science: Accurate measurements are fundamental in medical fields like drug dosage, clinical testing, and diagnosis. Inaccurate handling of significant figures can have serious health consequences.
-
Financial Analysis: Financial calculations also rely on precision, especially in investments and financial modeling. Errors in significant figures can lead to miscalculations of profit, loss, or investment returns.
Conclusion: Mastering Significant Figures for Accurate Results
Mastering significant figures is a cornerstone of scientific literacy and responsible data handling. It’s about more than just following rules; it's about understanding the inherent uncertainty in measurements and communicating that uncertainty accurately in our results. By carefully applying the rules outlined above and using tools like scientific notation, we can ensure our calculations are both precise and reflect the true precision of the measurements on which they are based. This precision isn't simply a matter of academic rigor; it has direct implications in numerous fields and directly impacts the safety and reliability of a vast range of applications. Neglecting significant figures can lead to potentially serious errors, highlighting the importance of consistently using and understanding this fundamental concept in measurement and calculation.
Latest Posts
Latest Posts
-
What Is The Mass Number Of An Atom Equal To
Mar 28, 2025
-
Le Chateliers Principle Lab Report Answers
Mar 28, 2025
-
How To Calculate Mass Of Excess Reactant
Mar 28, 2025
-
What Does Newtons First Law Say
Mar 28, 2025
-
Does Ionic Have High Boiling Point
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Valid Digits In A Measurement Are Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
