Vapour Pressure Of Water In Mmhg
Muz Play
Mar 29, 2025 · 6 min read
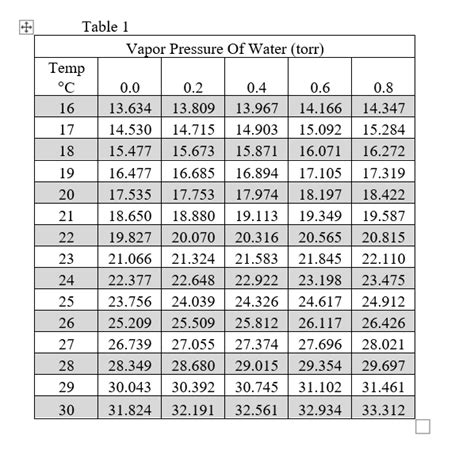
Table of Contents
Vapor Pressure of Water in mmHg: A Comprehensive Guide
Understanding the vapor pressure of water is crucial in numerous scientific and engineering disciplines, from meteorology and chemistry to HVAC systems and industrial processes. This comprehensive guide delves deep into the concept of water vapor pressure, specifically focusing on its measurement in millimeters of mercury (mmHg), exploring its dependence on temperature, and highlighting its practical applications.
What is Vapor Pressure?
Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. In simpler terms, it's the pressure exerted by the gaseous phase of a substance when it's in equilibrium with its liquid or solid phase. This equilibrium implies that the rate of evaporation equals the rate of condensation. For water, it's the pressure exerted by water vapor above a liquid water surface in a sealed container. When the number of water molecules escaping the liquid surface equals the number of water molecules returning to the liquid surface, a state of equilibrium is reached. This equilibrium pressure is the vapor pressure.
Water's Unique Properties and Vapor Pressure
Water exhibits unique properties compared to other liquids, influencing its vapor pressure behavior. Its strong hydrogen bonding results in relatively high boiling point and a relatively lower vapor pressure at a given temperature compared to substances with weaker intermolecular forces. This relatively lower vapor pressure is significant because it dictates how readily water evaporates.
Vapor Pressure of Water in mmHg: The Temperature Dependence
The vapor pressure of water is highly temperature-dependent. As temperature increases, the kinetic energy of water molecules increases, leading to a higher rate of evaporation. More water molecules escape into the gaseous phase, thus increasing the vapor pressure. Conversely, at lower temperatures, the vapor pressure decreases because fewer molecules have sufficient energy to overcome the intermolecular forces holding them in the liquid phase.
This relationship is not linear; it's accurately described by empirical equations or presented in tabular form. Several different equations exist, each with its own level of accuracy over a specific temperature range. One commonly used equation is the Antoine equation, which offers a good approximation:
log₁₀(P) = A - B/(T + C)
Where:
- P is the vapor pressure in mmHg
- T is the temperature in degrees Celsius
- A, B, and C are empirical constants specific to the substance (water, in this case). Different sets of constants might provide better accuracy across different temperature ranges.
The values of A, B, and C for water vary depending on the source and the desired temperature range. It's essential to use the constants applicable to the specific temperature range in question for accurate results.
Vapor Pressure Tables: A Practical Resource
Instead of using complex equations, vapor pressure tables provide readily accessible data. These tables list vapor pressure values (in mmHg or other units) corresponding to different temperatures. This is a highly convenient method for determining vapor pressure, especially for quick estimations.
Measuring Vapor Pressure of Water
Measuring the vapor pressure of water requires specialized equipment and techniques depending on the desired accuracy and temperature range. Several methods are available:
-
Manometric Methods: These methods involve using a manometer (a pressure-measuring instrument) to directly measure the pressure exerted by the water vapor in a closed system. Different types of manometers, like mercury manometers, are used for different pressure ranges and accuracy requirements.
-
Static Methods: These methods involve sealing a known amount of water in a closed container at a specific temperature and then measuring the pressure exerted by the water vapor using a pressure transducer or other pressure-measuring device.
-
Dynamic Methods: These methods involve measuring the pressure of a flowing water vapor stream, which is often more complex to set up and operate.
Applications of Water Vapor Pressure
The concept of vapor pressure of water has far-reaching applications in numerous fields:
1. Meteorology and Climate Science
-
Humidity: The partial pressure of water vapor in the atmosphere is a crucial factor in determining relative humidity. Relative humidity, a measure of how much water vapor is present in the air compared to its saturation capacity, directly relates to the vapor pressure of water at a given temperature. High relative humidity indicates the air is close to saturation, leading to condensation and the formation of clouds or fog.
-
Weather Forecasting: Understanding water vapor pressure helps predict weather patterns, including precipitation, fog formation, and dew point.
2. Chemical Engineering and Industrial Processes
-
Drying Processes: The vapor pressure of water determines the driving force for evaporation in drying processes. Understanding and controlling the vapor pressure is essential for efficient drying of various materials, such as food, chemicals, and pharmaceuticals.
-
Distillation: In distillation processes, the vapor pressure difference between different components of a mixture is used to separate them. The vapor pressure of water plays a critical role in the separation of water from other liquids.
-
HVAC Systems: The vapor pressure of water is crucial in designing and operating heating, ventilation, and air conditioning (HVAC) systems. Accurate control of humidity levels, directly related to the water vapor pressure, ensures optimal comfort and prevents issues like condensation.
3. Biology and Environmental Science
-
Plant Physiology: The vapor pressure of water plays a critical role in plant transpiration (water loss through stomata). Understanding water vapor pressure gradients between the leaf and the atmosphere is vital in understanding plant water relations.
-
Water Management: In managing water resources, understanding evaporation rates, directly influenced by the vapor pressure of water, is essential for irrigation scheduling and water conservation strategies.
Factors Affecting Vapor Pressure Beyond Temperature
While temperature is the most significant factor affecting the vapor pressure of water, other factors can also play a role:
-
Presence of Solutes: Dissolving solutes in water reduces its vapor pressure. This phenomenon, known as Raoult's Law, is due to the solute molecules occupying space at the liquid-vapor interface, thus reducing the number of water molecules that can escape into the gaseous phase. The extent of vapor pressure reduction depends on the concentration of the solute.
-
Atmospheric Pressure: While less significant than temperature, the total atmospheric pressure can influence the vapor pressure of water. Higher atmospheric pressure slightly suppresses the vapor pressure.
-
Presence of other gases: The presence of other gases in the air above the water can slightly affect the partial pressure of water vapor, although the effect is often negligible in comparison to temperature.
Conclusion
The vapor pressure of water, expressed in mmHg, is a fundamental concept with wide-ranging applications in various scientific and engineering fields. Its strong temperature dependence dictates its behavior and impacts numerous natural processes and industrial operations. Accurate determination and understanding of water vapor pressure, using various methods and resources like vapor pressure tables or equations, are essential for effective analysis and control in numerous applications, from weather forecasting to industrial process optimization. Further research into precise measurement techniques and a deeper understanding of the interactions between water and other components will continue to improve our understanding and ability to leverage this crucial property of water.
Latest Posts
Latest Posts
-
Modeling How Dna Fingerprints Are Made
Apr 01, 2025
-
Where Is Genetic Information Of The Cell Stored
Apr 01, 2025
-
The Amount Of Matter In An Object Is
Apr 01, 2025
-
What Is The Amdr For Fat For Adults
Apr 01, 2025
-
What Is The Difference Between A Closed And Open System
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Vapour Pressure Of Water In Mmhg . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
