What Is The Difference Between A Closed And Open System
Muz Play
Apr 01, 2025 · 6 min read
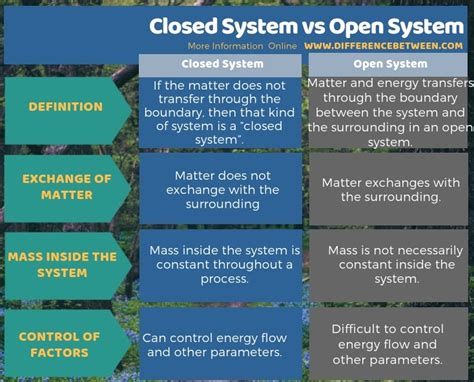
Table of Contents
What's the Difference Between a Closed and Open System? A Deep Dive
The terms "closed system" and "open system" are frequently encountered across various scientific disciplines, from physics and chemistry to ecology and computer science. While seemingly simple concepts, understanding the nuances of these classifications is crucial for comprehending complex processes and interactions within various systems. This article will delve into the fundamental differences between closed and open systems, exploring their characteristics, providing real-world examples, and highlighting the implications of these classifications in different fields.
Defining Open and Closed Systems: The Core Distinction
At its heart, the difference lies in the exchange of matter and energy with the surrounding environment.
Closed systems, in their purest form, do not exchange matter with their surroundings. This means no matter enters or leaves the system's boundaries. However, they can exchange energy with their environment. This energy exchange can take many forms, including heat, light, or work.
Open systems, on the other hand, exchange both matter and energy with their surroundings. Matter can freely flow in and out of the system, and energy transfer is also possible. This constant exchange is what distinguishes open systems and makes them dynamic and adaptable.
Exploring the Characteristics of Closed Systems
Closed systems are often characterized by their relative isolation from external influences. While energy can flow, the lack of matter exchange simplifies analysis in many cases. Let’s explore some key characteristics:
1. Limited Interaction with the Environment:
The defining feature is the restriction of matter transfer. This simplifies modeling since fewer variables need to be considered. Think of a perfectly sealed container – no matter can enter or leave, even if heat is allowed to transfer.
2. Predictable Behavior (Under Certain Conditions):
In many scenarios, closed systems exhibit more predictable behavior compared to open systems. If the initial conditions are known, and energy exchange is understood, the system's future state can often be predicted with reasonable accuracy, at least in principle. This is particularly true in fields like thermodynamics.
3. Potential for Equilibrium:
A closed system might eventually reach a state of equilibrium, where the distribution of energy and any internal components remains relatively constant over time. This equilibrium is often driven by the tendency towards maximum entropy (disorder).
4. Simplified Modeling:
The limited interaction facilitates simpler mathematical models. The absence of matter exchange reduces the complexity of equations used to describe system behavior.
Real-World Examples of Closed Systems (Approximations)
It’s important to remember that truly perfect closed systems rarely exist in nature. Most systems are open to some degree. However, some systems closely approximate closed systems under specific conditions:
- A sealed thermos: While some heat transfer inevitably occurs, it's minimized, making it a reasonable approximation of a closed system for maintaining temperature.
- A closed chemical reaction vessel: In a controlled laboratory setting, a sealed reaction vessel can approximate a closed system, allowing scientists to study chemical reactions without external interference.
- An adiabatic process (in thermodynamics): This refers to a process where no heat transfer occurs between the system and its surroundings. While matter exchange might occur, the absence of heat transfer leads to simplified calculations.
- A perfectly insulated room (theoretical): In theory, a room completely sealed and perfectly insulated could approximate a closed system regarding matter and, to a large degree, energy.
Delving into the Characteristics of Open Systems
Open systems are the norm in the natural world, demonstrating complexity and dynamic behavior due to the continuous exchange of matter and energy. Key characteristics include:
1. Constant Matter and Energy Exchange:
The fundamental feature is the continuous flow of both matter and energy across the system's boundaries. This constant interaction makes open systems highly dynamic and susceptible to external influences.
2. Dynamic Equilibrium:
Unlike closed systems which may reach static equilibrium, open systems tend towards a dynamic equilibrium, where matter and energy flow continually, maintaining a relatively stable state despite constant changes.
3. Adaptation and Self-Organization:
Open systems often demonstrate self-organization and adaptation to changing conditions. This ability to adapt is facilitated by the constant influx of resources and the expulsion of waste products.
4. Complexity and Emergence:
Open systems frequently exhibit emergent properties – characteristics that arise from the interaction of individual components but are not present in the individual components themselves. This complexity makes their behavior more difficult to predict compared to closed systems.
5. High degree of unpredictability:**
The constant influx of variables makes the long term behaviour of an open system difficult, if not impossible to accurately predict.
Real-World Examples of Open Systems
Open systems are prevalent in the natural world and human-created systems. Here are a few examples:
- An ecosystem (e.g., a forest): Forests exchange matter (nutrients, water, organisms) and energy (sunlight) with their surroundings. The constant flow of resources shapes the ecosystem's structure and dynamics.
- The human body: The body constantly exchanges matter (food, water, oxygen) and energy with its environment. Metabolic processes are driven by energy intake, and waste products are expelled.
- A business: Businesses exchange goods, services, money, and information with their customers, suppliers, and the wider economy. Their success depends on this constant exchange.
- A river: A river exchanges water and sediment with its surroundings as it flows. Changes in the river's flow, temperature, and composition are influenced by these interactions.
- The Earth's climate system: The Earth continuously exchanges energy (radiation from the sun) and matter (water vapor, gases) with space, affecting weather patterns and global climate.
The Implications of System Classifications
Understanding whether a system is open or closed has significant implications across many fields:
In Ecology: The classification helps model ecosystem dynamics, resource management, and conservation efforts. Open systems demand consideration of external factors influencing the ecosystem's health.
In Engineering: Open and closed system considerations are critical in designing and optimizing systems, such as chemical reactors, power plants, and control systems. Understanding material and energy flows is key to efficiency and safety.
In Economics: Economic models often treat economies as open systems, recognizing their interaction with the global economy and the impact of international trade.
In Biology: Living organisms are quintessential open systems, constantly exchanging matter and energy with their environments. Understanding this exchange is fundamental to studying metabolism, growth, and homeostasis.
Beyond the Binary: A Spectrum of Openness
While the terms "open" and "closed" provide a useful framework, it's important to recognize that these are not absolute categories. Many systems fall somewhere along a continuum of openness. A system might be largely closed in one aspect but open in others. For instance, a greenhouse might be largely closed in terms of matter exchange but open in terms of energy exchange (sunlight and heat). The degree of openness significantly impacts the complexity and predictability of the system.
Conclusion: Openness, Complexity, and Understanding
The distinction between open and closed systems is a fundamental concept with wide-ranging applications. While closed systems offer a simplified model with potentially predictable behavior, open systems, with their constant exchange of matter and energy, represent the reality of most natural and human-constructed systems. Understanding the characteristics and implications of these classifications is crucial for developing accurate models, making informed decisions, and addressing complex challenges across diverse fields. The ability to analyze the degree of openness in a given system is key to understanding its dynamics and behavior, paving the way for more effective problem-solving and informed predictions about the future. Further exploration of these principles will continue to drive advancements in various disciplines, allowing us to better manage and interact with the complex world around us.
Latest Posts
Latest Posts
-
What Is The Difference Between Ethnic And Religious Groups
Apr 02, 2025
-
Explain The Difference Between An Autotroph And A Heterotroph
Apr 02, 2025
-
Is Magnesium A Metal Nonmetal Or Metalloid
Apr 02, 2025
-
Number Of Atoms In A Simple Cubic Unit Cell
Apr 02, 2025
-
Inscribed Circle In A Right Triangle
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between A Closed And Open System . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
