Water Boiling Physical Or Chemical Change
Muz Play
Apr 01, 2025 · 5 min read
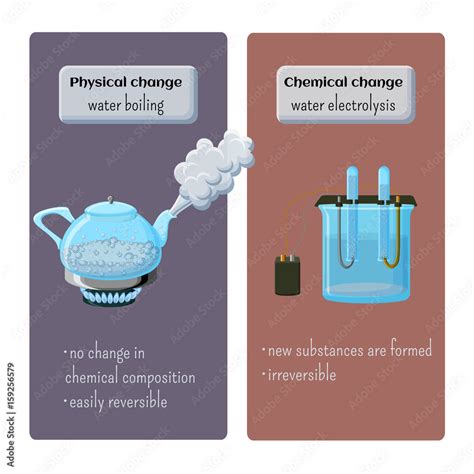
Table of Contents
Is Boiling Water a Physical or Chemical Change? A Deep Dive
The question of whether boiling water represents a physical or chemical change is a common one, especially in science education. While seemingly straightforward, a thorough understanding requires exploring the nuances of matter, its states, and the transformations it undergoes. This article will delve into the intricacies of boiling water, examining the process from a molecular perspective and clarifying its classification within the realm of physical and chemical changes. We will also explore related concepts and address common misconceptions.
Understanding Physical and Chemical Changes
Before tackling the boiling water conundrum, let's establish a clear understanding of the fundamental difference between physical and chemical changes.
Physical Changes: A Change in Form, Not Substance
A physical change alters the form or appearance of a substance without changing its chemical composition. Think of it as rearranging the furniture in a room – the furniture (the substance) remains the same, just in a different arrangement. Examples include:
- Changes of state: Melting ice (solid to liquid), boiling water (liquid to gas), freezing water (liquid to solid), and sublimation (solid to gas, like dry ice).
- Dissolving: Salt dissolving in water. The salt is still present; it's just dispersed in the water.
- Cutting, bending, or crushing: These actions change the shape of an object but don't alter its chemical makeup.
Chemical Changes: A Change in Composition
A chemical change, also known as a chemical reaction, involves a transformation that alters the chemical composition of a substance. New substances with different properties are formed. It's like taking the furniture apart and using the pieces to build something entirely new. Examples include:
- Burning: Combustion reactions produce new substances like carbon dioxide and water.
- Rusting: Iron reacts with oxygen to form iron oxide (rust), a completely different substance.
- Cooking: Many cooking processes involve chemical reactions, such as denaturation of proteins in eggs.
- Digestion: The breakdown of food in our bodies involves numerous chemical reactions.
Boiling Water: A Detailed Analysis
Now, let's apply these definitions to the process of boiling water. When water boils, it transitions from its liquid state to its gaseous state (water vapor or steam). This phase change occurs when the water molecules absorb enough energy (heat) to overcome the intermolecular forces holding them together in the liquid phase. The molecules gain kinetic energy, moving faster and further apart, eventually escaping into the gaseous phase.
Crucially, the chemical formula of water remains H₂O throughout the process. No new substances are formed. The water molecules are still composed of two hydrogen atoms and one oxygen atom. The only change is their state of aggregation and the distance between them. Therefore, boiling water is unequivocally a physical change.
Microscopic Perspective: What Happens at the Molecular Level?
To solidify our understanding, let's examine the process at a microscopic level. Liquid water consists of H₂O molecules constantly moving and interacting via intermolecular forces like hydrogen bonds. These bonds are relatively weak compared to the covalent bonds within each water molecule.
As heat is applied, the kinetic energy of the water molecules increases. This increased energy allows them to overcome the intermolecular forces, breaking free from the liquid structure and entering the gaseous phase as water vapor. The molecules themselves remain intact; their chemical structure hasn't changed.
Common Misconceptions About Boiling Water
Despite the seemingly simple nature of this phenomenon, several misconceptions persist:
-
Belief that boiling water decomposes: Some individuals mistakenly believe that boiling water decomposes into hydrogen and oxygen. This is incorrect. While electrolysis can decompose water into its constituent elements using an electric current, simply boiling water does not achieve this. The covalent bonds within the H₂O molecule are much stronger than the energy provided by heating.
-
Confusing evaporation with boiling: Evaporation is a similar phase transition but occurs at temperatures below the boiling point. It happens at the surface of a liquid, while boiling involves the formation of bubbles throughout the liquid. Both are physical changes, though.
-
Ignoring the dissolved substances: Tap water or other solutions contain dissolved minerals and impurities. Boiling may cause some changes in the concentration of these substances due to evaporation, but the fundamental process remains a physical change. The salts or other dissolved components remain chemically unchanged.
Beyond Boiling: Other Phase Transitions in Water
Water exhibits several other phase transitions, all classified as physical changes:
- Melting: The transition from solid ice to liquid water.
- Freezing: The transition from liquid water to solid ice.
- Sublimation: The transition directly from solid ice to water vapor (e.g., frost disappearing on a cold day).
- Deposition: The transition directly from water vapor to solid ice (e.g., frost formation).
In each case, the chemical composition of the water remains unchanged; only the arrangement and energy of the molecules are altered.
Implications and Applications
Understanding that boiling water is a physical change has numerous implications across various fields:
- Cooking: Boiling is a crucial method in cooking, preserving the nutritional value of ingredients while changing their texture.
- Water purification: While boiling doesn't remove all contaminants, it kills many harmful microorganisms, making water safer for consumption.
- Steam generation: Boiling water generates steam, which is used in various industrial processes and power generation.
- Distillation: Boiling and condensation are fundamental steps in water distillation, a purification process that separates water from impurities.
Conclusion: Boiling Water – A Physical Transformation
In summary, boiling water is definitively a physical change. The chemical composition of the water (H₂O) remains unchanged throughout the process. Only the physical state of the water transforms from liquid to gas. This seemingly simple phenomenon illustrates the important distinction between physical and chemical changes and highlights the fundamental principles of matter and its transformations. Understanding this distinction is crucial for grasping various scientific concepts and their practical applications in everyday life and numerous industries. The molecular level analysis underscores the integrity of the water molecule during boiling, emphasizing the fact that it's a phase transition, not a decomposition reaction. The misconceptions surrounding boiling water are addressed, further clarifying the precise nature of this common physical process. The broader context of other phase transitions in water reinforces the consistency of water's chemical identity throughout these transformations. Finally, the discussion of implications and applications showcases the significance of understanding this fundamental physical change in various real-world contexts.
Latest Posts
Latest Posts
-
What Is The Conflict Of The Play
Apr 02, 2025
-
The Cell Is The Basic Unit Of
Apr 02, 2025
-
Comparison Of Somatic And Autonomic Nervous Systems Concept Map
Apr 02, 2025
-
How To Convert Molecules To Atoms
Apr 02, 2025
-
Ejemplo De Diagrama De Cuerpo Libre
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Water Boiling Physical Or Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
