Water Is Pure Substance Or Mixture
Muz Play
Mar 22, 2025 · 4 min read
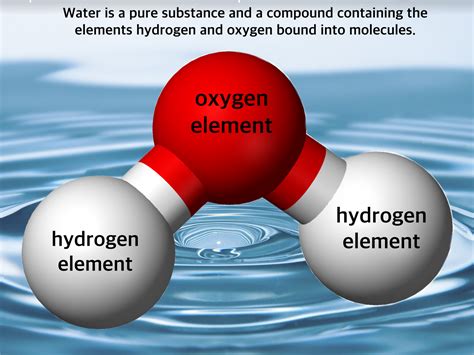
Table of Contents
Is Water a Pure Substance or a Mixture? A Deep Dive into the Chemistry of H₂O
The question of whether water is a pure substance or a mixture often sparks debate, especially among students new to chemistry. While seemingly simple, the answer requires a nuanced understanding of chemical definitions and the complexities of natural water sources. This comprehensive article will delve deep into the chemical composition of water, exploring its properties and clarifying its classification. We’ll examine different types of water and dispel common misconceptions surrounding its purity. By the end, you'll have a solid grasp of why water's classification isn't as straightforward as it might initially appear.
Defining Pure Substances and Mixtures
Before we tackle the main question, let's establish clear definitions.
Pure Substance: A pure substance is a form of matter that has a constant composition (it's made of only one type of atom or molecule) and distinct chemical properties. It cannot be separated into simpler substances by physical methods. Examples include elements (like oxygen or gold) and compounds (like water or salt).
Mixture: A mixture is a combination of two or more substances that are not chemically bonded. Each substance retains its individual chemical properties, and mixtures can be separated by physical methods like filtration, distillation, or evaporation. Mixtures are further categorized into homogeneous (uniform composition throughout) and heterogeneous (non-uniform composition).
The Chemical Composition of Water: A Pure Compound
Chemically, water is a pure substance, specifically a compound. It's composed of two elements, hydrogen and oxygen, chemically bonded in a fixed ratio of 2:1. This means that every water molecule (H₂O) always contains two hydrogen atoms covalently bonded to one oxygen atom. This fixed ratio and the strong chemical bond distinguish it from a mixture. You cannot physically separate hydrogen and oxygen from water without a chemical reaction (like electrolysis).
Different Types of Water: Purity in Perspective
While pure water (H₂O) is a compound, the water we encounter in daily life rarely exists in this perfectly pure state. This is where the complexity arises. The "purity" of water depends on the context and what impurities are considered significant.
1. Distilled Water: Approaching Purity
Distilled water is the closest we can get to pure H₂O in practical terms. The distillation process involves boiling water and then condensing the steam, leaving behind dissolved solids and other impurities. While incredibly pure compared to other sources, even distilled water may contain trace amounts of dissolved gases from the atmosphere.
2. Deionized Water: Removing Ions
Deionized water undergoes a process that removes ions (charged particles) like minerals and salts. This method is often used in scientific labs and industrial settings where the presence of ions can interfere with experiments or processes. Like distilled water, it's not entirely devoid of impurities, but the ionic content is significantly reduced.
3. Tap Water: A Mixture
Tap water is a classic example of a mixture. It contains various dissolved minerals (like calcium and magnesium), dissolved gases (like oxygen and carbon dioxide), and potentially trace amounts of organic matter and pollutants. The exact composition varies greatly depending on the source and the water treatment process. This variability highlights why tap water is considered a mixture rather than a pure substance.
4. Seawater: A Complex Mixture
Seawater is a highly complex mixture containing a large quantity of dissolved salts (primarily sodium chloride), as well as various other minerals, organic matter, and microorganisms. Its high salinity makes it unsuitable for drinking without desalination.
5. Groundwater: Variable Composition
Groundwater’s composition also depends significantly on the geology of the area. It can contain dissolved minerals, gases, and potentially pollutants, making it a mixture with variable characteristics.
Understanding the Misconception
The confusion often stems from the fact that "pure water" in everyday language doesn't always align with the strict chemical definition. We often use "pure" to describe water that is clean, safe to drink, and free from harmful contaminants. However, this doesn't necessarily mean it's chemically pure H₂O.
The Importance of Water Purity
The purity of water is crucial for various applications.
-
Drinking Water: Safe drinking water requires the removal of harmful bacteria, viruses, and pollutants. While perfect purity isn't necessary, standards exist to ensure health and safety.
-
Industrial Processes: Many industrial processes demand highly pure water to prevent contamination or undesirable reactions.
-
Scientific Research: Experiments often rely on high-purity water to avoid interfering with the results.
-
Medical Applications: Sterile, pure water is crucial in medical settings.
Conclusion: Water – A Pure Compound in an Impure World
In summary, water (H₂O) is a pure substance, specifically a compound. It’s composed of a fixed ratio of hydrogen and oxygen atoms chemically bonded together. However, the water we encounter in our daily lives is rarely pure in the strictest chemical sense. Tap water, seawater, and groundwater are all mixtures containing various dissolved substances and impurities. Understanding this distinction is crucial for appreciating the complexities of water chemistry and the significance of water purity in different contexts. The "purity" of water is highly contextual, ranging from the nearly pure distilled water used in labs to the complex mixtures found in natural water sources. This understanding is essential for various applications, from ensuring safe drinking water to conducting accurate scientific research.
Latest Posts
Latest Posts
-
What Are Two Kinds Of Pure Substances
Mar 23, 2025
-
What Is The Smallest Particle In An Element
Mar 23, 2025
-
Gas Laws Practice Problems And Answers
Mar 23, 2025
-
Is Carbonate Ion A Strong Base
Mar 23, 2025
-
What Is A Family Of Elements
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Water Is Pure Substance Or Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
