What Is A Family Of Elements
Muz Play
Mar 23, 2025 · 7 min read
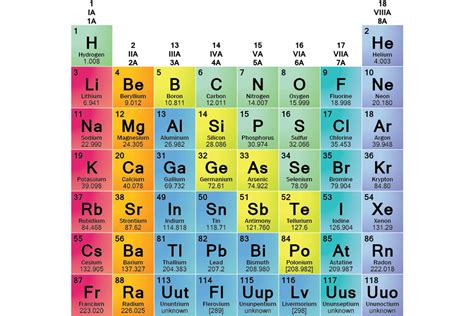
Table of Contents
What is a Family of Elements? Understanding Periodic Trends and Group Properties
The periodic table, a cornerstone of chemistry, organizes elements not randomly, but based on their recurring properties and electronic configurations. Instead of viewing elements in isolation, understanding them within the context of their "families" – also known as groups or columns – provides crucial insights into their chemical behavior, reactivity, and physical properties. This article delves deep into the concept of element families, exploring their defining characteristics, periodic trends within families, and the practical implications of this organizational system.
Defining Element Families: A Shared Electron Configuration
Element families are vertical columns on the periodic table. Each family comprises elements with similar outermost electron shell configurations. This similarity in electron configuration is the fundamental reason why elements within a family exhibit similar chemical properties. The outermost electrons, known as valence electrons, are primarily responsible for the formation of chemical bonds and dictate an element's reactivity. Elements in the same family have the same number of valence electrons, leading to predictable patterns in their behavior.
For instance, the alkali metals (Group 1) all possess one valence electron. This single electron readily participates in chemical reactions, making alkali metals highly reactive. Similarly, the halogens (Group 17) possess seven valence electrons, one electron short of a stable octet. This electron deficiency makes halogens highly reactive as they readily gain an electron to achieve a stable configuration.
The Significance of Valence Electrons
Valence electrons are the key to understanding element families. Their number determines:
- Oxidation state: The charge an element carries when it forms a chemical bond. Elements in the same family often exhibit similar oxidation states.
- Bonding behavior: Whether an element prefers to form ionic bonds (transferring electrons), covalent bonds (sharing electrons), or metallic bonds (delocalized electrons).
- Reactivity: How readily an element participates in chemical reactions. Elements with fewer or more valence electrons than a stable octet tend to be more reactive.
- Physical properties: While not as directly related as chemical properties, valence electrons influence properties such as melting point, boiling point, and electrical conductivity.
Exploring Key Element Families: A Detailed Look
Let's delve into some of the most significant element families and examine their characteristic properties:
1. Alkali Metals (Group 1): The Reactive Lone Wolves
The alkali metals – lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr) – are known for their exceptional reactivity. Their single valence electron is easily lost, resulting in the formation of +1 ions. This explains their low ionization energies and high electronegativities. Alkali metals are soft, silvery-white metals, and their reactivity increases as you move down the group. They readily react with water, producing hydrogen gas and a metal hydroxide.
Key Properties of Alkali Metals:
- Highly reactive: React vigorously with water and air.
- Low melting and boiling points: Compared to other metals.
- Soft: Easily cut with a knife.
- Good conductors of electricity and heat: Due to their loosely held valence electron.
- Form +1 ions: readily lose their single valence electron.
2. Alkaline Earth Metals (Group 2): The Moderately Reactive Duo
Alkaline earth metals – beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra) – possess two valence electrons. They are less reactive than alkali metals because losing two electrons requires more energy. However, they still readily participate in chemical reactions, forming +2 ions. They are harder, denser, and have higher melting points than alkali metals.
Key Properties of Alkaline Earth Metals:
- Moderately reactive: Less reactive than alkali metals.
- Higher melting and boiling points: Than alkali metals.
- Harder: Than alkali metals.
- Good conductors of electricity and heat: but less so than alkali metals.
- Form +2 ions: readily lose their two valence electrons.
3. Halogens (Group 17): The Electron-Hungry Seekers
Halogens – fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At) – are highly reactive nonmetals. With seven valence electrons, they are one electron short of a stable octet. This drives their strong tendency to gain an electron, forming -1 ions. Their reactivity decreases down the group, with fluorine being the most reactive halogen.
Key Properties of Halogens:
- Highly reactive: Tend to gain one electron to form -1 ions.
- Nonmetals: Lack metallic properties.
- Diatomic molecules: Exist as diatomic molecules (e.g., F₂, Cl₂) in their elemental form.
- Varied physical states: Fluorine and chlorine are gases, bromine is a liquid, and iodine is a solid.
- Form -1 ions: readily gain one electron to achieve a stable octet.
4. Noble Gases (Group 18): The Inert Giants
Noble gases – helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn) – are famously unreactive. They possess a full outer electron shell (eight valence electrons, except for helium with two), making them exceptionally stable. This explains their low reactivity and the fact that they rarely form compounds. They are all gases at room temperature.
Key Properties of Noble Gases:
- Inert: Very low reactivity.
- Gases at room temperature: Due to their weak interatomic forces.
- Full outer electron shell: Eight valence electrons (except helium).
- High ionization energies: Requires significant energy to remove an electron.
- Low boiling points: Weak interatomic forces result in low boiling points.
Periodic Trends within Element Families
Understanding element families also involves recognizing periodic trends. These are systematic changes in properties as you move down or across the periodic table. Within a family:
- Atomic radius increases down the group: As you add electron shells, the atomic size increases.
- Ionization energy decreases down the group: It becomes easier to remove an electron as the outermost electron is farther from the nucleus.
- Electronegativity decreases down the group: The attraction for electrons decreases as the outermost electrons are farther from the nucleus.
Practical Implications of Understanding Element Families
The concept of element families is not merely an academic exercise. It has significant practical implications in various fields:
- Predicting chemical reactions: Knowing the family of an element allows chemists to predict how it will react with other substances.
- Designing new materials: Understanding the properties of element families helps in designing new materials with specific properties. For instance, understanding the properties of alkali metals is crucial in developing high-performance batteries.
- Environmental science: The behavior of elements in the environment is often related to their family. Understanding this behavior is crucial for environmental remediation and pollution control.
- Medicine: The properties of elements within families are crucial in developing pharmaceuticals. Understanding how elements interact within the body is vital for drug development.
- Industrial applications: Many industrial processes rely on the unique properties of specific element families.
Beyond the Main Group Families: Transition Metals and Inner Transition Metals
While the main group elements (Groups 1-18) are the most straightforward examples of element families, the periodic table also includes transition metals and inner transition metals (lanthanides and actinides). These elements exhibit more complex behavior due to the involvement of d and f orbitals in their electronic configurations. While their properties aren't as uniformly predictable as the main group elements, they still show similarities within their respective series.
Transition Metals: Exhibit variable oxidation states, often forming colored compounds, and are frequently used as catalysts.
Inner Transition Metals: Lanthanides and actinides are characterized by the filling of their 4f and 5f orbitals, respectively. They show a high degree of similarity in their chemical properties due to the shielding effect of the electrons in these orbitals.
Conclusion: The Power of Organization
The concept of element families offers a powerful framework for understanding the chemical behavior and properties of elements. By recognizing the similarities within families, based on their shared valence electron configurations, we can make accurate predictions about reactivity, bonding, and physical properties. This knowledge is essential for advancements in various scientific fields, including chemistry, materials science, environmental science, and medicine. The periodic table, with its elegantly organized families of elements, remains a testament to the underlying order and predictability within the seemingly diverse world of chemical elements. Further exploration of individual families and their nuanced properties continues to reveal new insights and applications for this fundamental organizational system.
Latest Posts
Latest Posts
-
The Rows In The Periodic Table Are Called
Mar 24, 2025
-
How To Determine Profit Maximizing Price
Mar 24, 2025
-
General Chemistry Principles And Modern Applications Petrucci
Mar 24, 2025
-
Osmosis And Diffusion Lab With Dialysis Tubing Answers
Mar 24, 2025
-
Does Water Have A Negative Charge
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about What Is A Family Of Elements . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
