Does Water Have A Negative Charge
Muz Play
Mar 24, 2025 · 5 min read
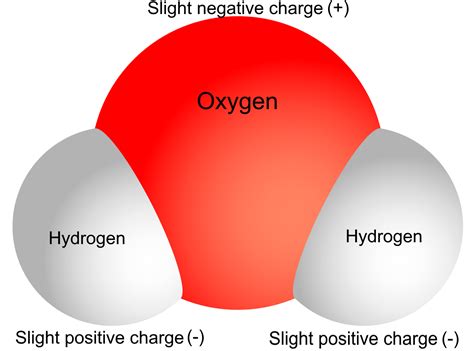
Table of Contents
Does Water Have a Negative Charge? Delving into the Polarity and Charge Distribution of H₂O
The question of whether water has a negative charge is more nuanced than a simple yes or no. While water itself is electrically neutral, its molecular structure leads to a fascinating distribution of charge that significantly impacts its properties and behavior. Understanding this requires exploring the concepts of polarity, electronegativity, and hydrogen bonding. This article will delve deep into the intricacies of water's charge distribution, examining common misconceptions and clarifying the true nature of its electrical properties.
Understanding Water's Molecular Structure: The Key to its Charge Distribution
Water (H₂O) is a simple molecule, yet its unique structure is the foundation of its remarkable properties. It consists of one oxygen atom covalently bonded to two hydrogen atoms. The crucial point here is the uneven distribution of electrons within this bond.
Electronegativity: The Heart of Polarity
Oxygen is significantly more electronegative than hydrogen. Electronegativity is an atom's ability to attract electrons within a chemical bond. Oxygen's higher electronegativity means it attracts the shared electrons in the O-H bonds more strongly than hydrogen does. This unequal sharing of electrons creates a polar covalent bond.
Polar Covalent Bonds: Creating Partial Charges
The result of this unequal electron sharing is that the oxygen atom develops a partial negative charge (δ-), while each hydrogen atom develops a partial positive charge (δ+). This is crucial: water doesn't have a net negative charge; instead, it possesses a polarity, a separation of charge within the molecule. It's this polarity that dictates much of water's behavior.
Visualizing the Polarity: The Bent Molecular Geometry
The molecular geometry of water further enhances its polarity. The two O-H bonds are not arranged linearly; instead, they form a bent shape with an angle of approximately 104.5 degrees. This bent structure prevents the partial positive and negative charges from canceling each other out. The overall molecule has a dipole moment, meaning it has a positive end and a negative end, like a tiny magnet.
The Role of Hydrogen Bonding: Amplifying Water's Interactions
The partial charges in water molecules are responsible for the formation of hydrogen bonds. These are relatively weak intermolecular forces that occur between the partially positive hydrogen atom of one water molecule and the partially negative oxygen atom of another.
Hydrogen Bonds: A Network of Interactions
Hydrogen bonds are responsible for many of water's unique properties, including its high boiling point, surface tension, and its ability to act as a universal solvent. These bonds create a complex, dynamic network between water molecules, influencing its overall behavior in many contexts.
Impact on Charge Distribution: A Collective Effect
While individual water molecules possess partial charges, the collective effect of hydrogen bonding means that the distribution of charge in bulk water is even more complex. The dynamic nature of these bonds constantly shifts the distribution of partial charges, affecting its interactions with other molecules and ions.
Debunking Misconceptions: Clarifying the Charge of Water
It's essential to clarify some common misconceptions surrounding water's charge:
Misconception 1: Water is Negatively Charged
This is incorrect. While water molecules have partial negative charges on their oxygen atoms, the overall molecule is electrically neutral. The positive and negative charges are balanced within each molecule. It’s the distribution of charge, not the net charge, that is significant.
Misconception 2: Pure Water is a Good Conductor of Electricity
Pure water is actually a very poor conductor of electricity. The ability of a substance to conduct electricity depends on the presence of free-moving charged particles (ions). Pure water contains very few ions, which is why it's a poor conductor. However, the presence of even small amounts of dissolved salts or other impurities dramatically increases its conductivity due to the increased number of ions.
Misconception 3: The Negative Charge Explains Water's Properties
While the partial negative charge on the oxygen atom plays a vital role in many of water's properties, it is not the sole explanation. The combination of polarity, hydrogen bonding, and the bent molecular geometry all work together to create the unique properties of water.
Water's Interactions with Other Charged Species: A Deeper Dive
Water's polarity significantly affects its interactions with other charged species:
Interactions with Ions: Solvation and Dissolution
Water's polar nature makes it an excellent solvent for ionic compounds. The partially positive hydrogen atoms attract the negative ions (anions), while the partially negative oxygen atoms attract the positive ions (cations). This process, called solvation, effectively surrounds and separates the ions, dissolving the ionic compound. This is why water is such a good solvent for many salts and minerals.
Interactions with Polar Molecules: Solubility and Hydrogen Bonding
Water also readily interacts with other polar molecules. The partial charges in these molecules interact with the partial charges in water molecules, leading to solubility. Furthermore, hydrogen bonds can form between water and other polar molecules, further strengthening these interactions.
Interactions with Nonpolar Molecules: Limited Solubility
Water's interaction with nonpolar molecules is limited. Nonpolar molecules lack significant charge separation, preventing strong interactions with water molecules. This explains why many nonpolar substances (like oil) are insoluble in water.
Conclusion: The Complexity of Water's Charge Distribution
In conclusion, the question of whether water has a negative charge requires a nuanced understanding of its molecular structure and interactions. While individual water molecules possess partial positive and negative charges due to the polarity of the O-H bonds, the overall molecule remains electrically neutral. The distribution of these partial charges, along with the significant role of hydrogen bonding, is what dictates water's exceptional properties as a solvent, a facilitator of life, and a fundamental component of our planet. Understanding this complex interplay of charge distribution is crucial for comprehending many natural phenomena and technological applications. The seemingly simple water molecule is, in fact, a remarkable testament to the power of subtle charge differences in dictating macroscopic behavior.
Latest Posts
Latest Posts
-
Difference Between Meiosis I And Ii
Mar 26, 2025
-
A Van Der Waals Interaction Is The Weak Attraction Between
Mar 26, 2025
-
Which Element Is Important In Directly Triggering Contraction
Mar 26, 2025
-
Is Salt Water An Element Compound Or Mixture
Mar 26, 2025
-
How To Find Change In Internal Energy
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Does Water Have A Negative Charge . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
