A Van Der Waals Interaction Is The Weak Attraction Between
Muz Play
Mar 26, 2025 · 6 min read
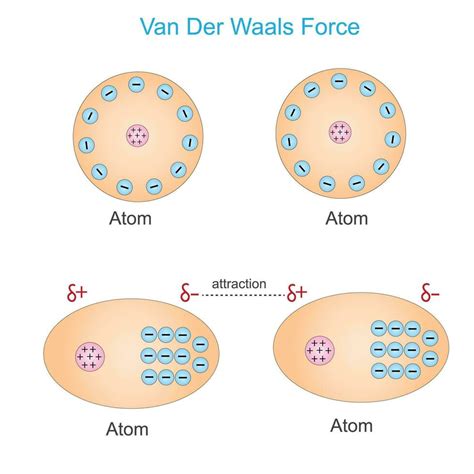
Table of Contents
A Van der Waals Interaction: The Weak Attraction Between Molecules
Van der Waals interactions are weak, short-range electrostatic attractive forces between molecules. While individually weak, their cumulative effect can be significant, influencing a wide range of physical and biological phenomena, from the gecko's ability to climb walls to the structure of proteins. Understanding these forces is crucial in various scientific disciplines, including chemistry, physics, and materials science. This article will delve deep into the nature of van der Waals interactions, exploring the different types, their origins, and their importance in diverse systems.
Understanding the Fundamentals: Types of Van der Waals Forces
Van der Waals forces are a collective term encompassing several distinct types of intermolecular attractions:
1. Keesom Forces (Orientation Forces):
These forces arise between permanent dipoles. Molecules possessing a permanent dipole moment (like water, with its polar O-H bonds) possess a positive and negative end. The positive end of one molecule is attracted to the negative end of another, leading to a net attractive force. The strength of Keesom forces is dependent on the magnitude of the dipole moments and the temperature; higher temperatures increase molecular motion, disrupting the alignment and weakening the interaction.
Key Characteristics:
- Origin: Interaction between permanent dipoles.
- Strength: Moderate, stronger than London dispersion forces but weaker than hydrogen bonds.
- Distance Dependence: Decreases rapidly with increasing distance (inverse sixth power).
- Examples: Interactions between water molecules, interactions between polar organic molecules.
2. Debye Forces (Induction Forces):
Debye forces occur between a permanent dipole and an induced dipole. A permanent dipole can induce a temporary dipole in a nearby molecule that is typically nonpolar. This happens because the electric field of the permanent dipole distorts the electron cloud of the nonpolar molecule, creating a temporary asymmetry in charge distribution. The permanent dipole is then attracted to this newly induced dipole.
Key Characteristics:
- Origin: Interaction between a permanent dipole and an induced dipole.
- Strength: Weaker than Keesom forces but stronger than London dispersion forces.
- Distance Dependence: Decreases rapidly with increasing distance (inverse sixth power).
- Examples: Interaction between a polar molecule (like HCl) and a nonpolar molecule (like methane).
3. London Dispersion Forces (Instantaneous Dipole-Induced Dipole Forces):
These are the most ubiquitous type of van der Waals interaction, present in all molecules, regardless of their polarity. Even nonpolar molecules like helium or methane experience these forces. They arise from temporary fluctuations in electron distribution within a molecule. At any given instant, the electrons might be momentarily clustered on one side of the molecule, creating a transient dipole. This instantaneous dipole can then induce a dipole in a neighboring molecule, leading to a weak attractive force. The larger and more easily polarizable the molecule (meaning its electron cloud is more easily distorted), the stronger the London dispersion forces.
Key Characteristics:
- Origin: Interaction between temporary, instantaneous dipoles.
- Strength: Weakest type of van der Waals force.
- Distance Dependence: Decreases rapidly with increasing distance (inverse sixth power).
- Examples: Interactions between nonpolar molecules like noble gases, interactions between hydrocarbons.
- Impact of Molecular Size and Shape: Larger molecules with greater surface area exhibit stronger London dispersion forces due to increased opportunities for instantaneous dipole interactions. Linear molecules generally have stronger dispersion forces than branched molecules of similar molar mass due to increased contact area.
The Significance of Van der Waals Interactions
While individually weak, the cumulative effect of van der Waals interactions can be substantial, playing a crucial role in various phenomena:
1. Biological Systems:
- Protein Folding: The intricate three-dimensional structures of proteins are largely determined by a delicate balance of different types of intermolecular forces, including van der Waals interactions. These weak forces contribute significantly to the stability and specific conformation of proteins, influencing their function.
- Enzyme-Substrate Interactions: The binding of a substrate to an enzyme often involves van der Waals forces, contributing to the specificity and efficiency of enzymatic reactions.
- DNA Base Pairing: The double helix structure of DNA is partly stabilized by van der Waals interactions between the stacked base pairs.
- Cell Adhesion: Many cell-cell and cell-substrate interactions rely on weak forces such as van der Waals interactions, enabling cell adhesion and tissue formation.
2. Materials Science:
- Adhesion: Many adhesives rely on van der Waals forces to create strong bonds between surfaces. The strength of the adhesive is affected by the surface area and the type of molecules involved.
- Liquefaction of Gases: The liquefaction of noble gases, which have no other intermolecular forces besides London dispersion forces, demonstrates the importance of these interactions at low temperatures.
- Surface Tension: The surface tension of liquids is partly a result of the attractive van der Waals forces between molecules at the surface.
- Crystal Structure: The packing of molecules in crystalline solids is influenced by van der Waals interactions, which determine the overall structure and properties of the material.
3. Physical Phenomena:
- Gecko Adhesion: The remarkable ability of geckos to climb walls is attributed to van der Waals forces between the tiny hairs on their feet and the surface they climb. The enormous number of these weak interactions results in a surprisingly strong adhesive force.
- Capillary Action: The movement of liquids in narrow tubes or porous materials, known as capillary action, is partly driven by van der Waals forces between the liquid molecules and the surface of the tube.
- Intermolecular Forces: Van der Waals forces are an example of an intermolecular force that greatly influences the physical properties of substances such as boiling points and melting points.
Comparing Van der Waals Forces to Other Intermolecular Forces
It's important to distinguish van der Waals forces from other intermolecular forces, particularly hydrogen bonds:
- Hydrogen Bonds: Are stronger than van der Waals forces. They arise from the interaction between a hydrogen atom bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) and a lone pair of electrons on another electronegative atom. Hydrogen bonds play a crucial role in the structure and properties of water and many biological molecules.
- Ion-Dipole Interactions: Occur between an ion and a polar molecule. These are stronger than typical van der Waals forces.
- Ion-Induced Dipole Interactions: Similar to Debye forces but involve an ion instead of a permanent dipole, resulting in stronger interactions.
Factors Affecting the Strength of Van der Waals Interactions
Several factors influence the strength of van der Waals interactions:
- Polarizability: The ease with which the electron cloud of a molecule can be distorted. Larger, more complex molecules are generally more polarizable and exhibit stronger London dispersion forces.
- Distance: The strength of van der Waals forces decreases rapidly with increasing distance between molecules. This is why they are considered short-range forces.
- Temperature: Higher temperatures increase molecular motion, which can disrupt the alignment of dipoles and weaken the interactions.
- Molecular Shape: The shape of molecules affects the extent of contact between them and consequently influences the strength of van der Waals forces. Linear molecules often have stronger interactions than branched molecules of similar molecular weight.
Conclusion: The Ubiquitous Influence of Weak Forces
Van der Waals interactions, though individually weak, are essential forces that shape the world around us. Their cumulative effect influences a vast array of phenomena, from the structure of biological macromolecules to the behavior of materials. Understanding these forces is crucial for advancing our knowledge in various scientific fields and developing new technologies. Further research into the intricacies of van der Waals interactions promises to uncover even more fascinating insights into the molecular world and its diverse manifestations. The seemingly insignificant attraction between molecules plays a surprisingly significant role in determining the properties and behavior of matter at all scales.
Latest Posts
Latest Posts
-
Punnett Squares Crosses Involving One Trait
Mar 29, 2025
-
When Pressure Increases Then The Volume Must
Mar 29, 2025
-
How To Check For Linear Independence
Mar 29, 2025
-
Find The Characteristic Polynomial Of The Matrix
Mar 29, 2025
-
Atoms That Gain Or Lose Electrons Are Called
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about A Van Der Waals Interaction Is The Weak Attraction Between . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
