When Pressure Increases Then The Volume Must
Muz Play
Mar 29, 2025 · 6 min read
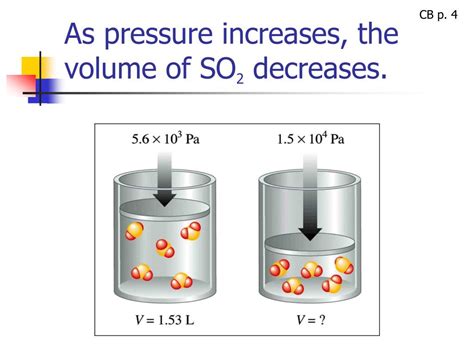
Table of Contents
When Pressure Increases, Then the Volume Must… Decrease: Understanding Boyle's Law
The relationship between pressure and volume of a gas has captivated scientists for centuries. Understanding this relationship is crucial in numerous fields, from designing scuba diving equipment to understanding the mechanics of the human respiratory system. The fundamental principle governing this interaction is known as Boyle's Law, which states that at a constant temperature, the volume of a gas is inversely proportional to its pressure. In simpler terms: when pressure increases, the volume must decrease, and vice versa. This article will delve into Boyle's Law, explaining its principles, applications, and limitations.
Understanding Boyle's Law: An Inverse Relationship
Boyle's Law, named after Robert Boyle, a 17th-century Irish chemist and physicist, is mathematically represented as:
P₁V₁ = P₂V₂
Where:
- P₁ represents the initial pressure
- V₁ represents the initial volume
- P₂ represents the final pressure
- V₂ represents the final volume
This equation signifies the inverse relationship between pressure and volume. If the pressure on a gas is doubled (P₂ = 2P₁), the volume will be halved (V₂ = V₁/2) to maintain the equality. Conversely, if the pressure is halved, the volume will double. This holds true as long as the temperature and the amount of gas remain constant.
The Molecular Explanation
To grasp Boyle's Law intuitively, consider the behavior of gas molecules at a microscopic level. Gas molecules are in constant, random motion, colliding with each other and the walls of their container. Pressure is essentially the force exerted by these gas molecules per unit area on the container walls.
-
Increased Pressure: When pressure increases, more gas molecules collide with the container walls per unit time and with greater force. To accommodate this increased impact, the gas molecules are forced closer together, resulting in a smaller volume.
-
Decreased Pressure: Conversely, when pressure decreases, the gas molecules experience fewer collisions with the container walls, and the impact force diminishes. This allows the gas molecules to spread out, occupying a larger volume.
Applications of Boyle's Law: From Scuba Diving to Breathing
Boyle's Law is not just a theoretical concept; it has far-reaching practical applications across various scientific and engineering disciplines.
1. Scuba Diving: Managing Pressure Changes Underwater
Scuba diving relies heavily on an understanding of Boyle's Law. As divers descend deeper into the water, the pressure exerted on their bodies increases significantly. This increase in pressure causes the air in their scuba tanks to compress, reducing its volume. Divers must therefore account for this compression when planning their dives, ensuring they have enough air to reach their destination and return safely. Conversely, as divers ascend, the pressure decreases, and the air in their lungs expands. Failing to manage this expansion can lead to serious lung injuries, highlighting the critical role of Boyle's Law in ensuring diver safety.
2. Respiratory System: Breathing Mechanics
The human respiratory system also operates based on principles similar to Boyle's Law. Inhalation involves increasing the volume of the lungs by contracting the diaphragm and intercostal muscles. This expansion creates a lower pressure within the lungs compared to the atmospheric pressure, causing air to rush into the lungs. Exhalation, on the other hand, involves relaxing the diaphragm and intercostal muscles, decreasing the lung volume and increasing the internal pressure, forcing air out of the lungs.
3. Meteorology: Weather Forecasting and Balloon Behavior
Boyle's Law plays a role in understanding atmospheric pressure changes and their impact on weather patterns. Changes in atmospheric pressure affect the volume of air masses, influencing weather systems and the behavior of weather balloons. These balloons are used for meteorological observations, and their altitude is heavily influenced by the pressure changes based on Boyle's Law.
4. Medical Applications: Respiratory Therapy and Anesthesia
In medicine, Boyle's Law is crucial in respiratory therapy, where it helps regulate the delivery of oxygen and other gases to patients with respiratory problems. It's also relevant in anesthesia, affecting the administration and effects of anesthetic gases.
5. Industrial Processes: Pneumatic Systems and Manufacturing
Industrial processes often utilize pneumatic systems that rely on the principles of Boyle's Law. Compressed air is used to power various machinery and tools, and understanding the pressure-volume relationship is essential for optimizing their performance and efficiency.
Deviations from Boyle's Law: When the Ideal Gas Law Takes Over
While Boyle's Law provides a good approximation of the relationship between pressure and volume for many gases under ordinary conditions, it's important to acknowledge its limitations. Boyle's Law is based on the ideal gas law, which assumes that gas molecules are point masses with negligible volume and that there are no intermolecular forces between them. This assumption holds well for gases at low pressures and high temperatures.
However, at high pressures and low temperatures, the intermolecular forces and the finite volume of gas molecules become significant, causing deviations from Boyle's Law. Under these conditions, the van der Waals equation provides a more accurate representation of the gas behavior. This equation accounts for both intermolecular forces and the finite volume of gas molecules, leading to more realistic predictions of pressure-volume relationships.
Factors Affecting Deviations from Boyle's Law
Several factors contribute to deviations from the ideal gas behavior predicted by Boyle's Law:
-
High Pressure: At high pressures, gas molecules are forced closer together, and the attractive intermolecular forces become more pronounced. This reduces the volume more than what Boyle's Law predicts.
-
Low Temperature: At low temperatures, the kinetic energy of gas molecules decreases, and the intermolecular attractive forces become more dominant. This also leads to deviations from Boyle's Law.
-
Polarity of Gas Molecules: The presence of polar molecules affects intermolecular forces, causing deviations from ideal behavior more prominently than non-polar molecules.
Conclusion: Boyle's Law and Beyond
Boyle's Law is a cornerstone of gas laws, providing a simple yet powerful description of the inverse relationship between pressure and volume of a gas at constant temperature. Its applications are widespread, ranging from scuba diving safety to industrial processes. While the ideal gas law underlying Boyle's Law provides a good approximation under many conditions, it's crucial to understand its limitations, especially at high pressures and low temperatures, where the van der Waals equation offers a more accurate model. A comprehensive understanding of Boyle's Law and its limitations is essential for anyone working with gases in any capacity, from scientific research to engineering applications. Further exploration into related gas laws, such as Charles's Law and the combined gas law, will provide a more complete understanding of gas behavior under various conditions. The study of gas laws remains a vital area of research, constantly refining our understanding of the physical world.
Latest Posts
Latest Posts
-
Which Is A Major Factor Affecting Population Growth Rate
Mar 31, 2025
-
Identify The Plant Tissues In The Three Images
Mar 31, 2025
-
Thesis Statement Examples For Literary Analysis
Mar 31, 2025
-
Why Do Atoms Form Bonds With Other Atoms
Mar 31, 2025
-
What Is The Law Of Conservation Of Charge
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about When Pressure Increases Then The Volume Must . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
