Atoms That Gain Or Lose Electrons Are Called
Muz Play
Mar 29, 2025 · 6 min read
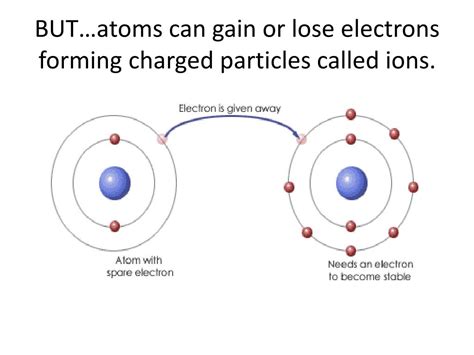
Table of Contents
Atoms That Gain or Lose Electrons Are Called Ions: A Deep Dive into Ionic Bonding and its Implications
Atoms are the fundamental building blocks of matter, tiny particles that make up everything around us. But what happens when these seemingly indivisible units interact? Often, they exchange or share electrons, the negatively charged particles orbiting the atom's nucleus. When an atom gains or loses electrons, it transforms into something new: an ion. Understanding ions is crucial to comprehending a vast array of chemical processes and the properties of many materials. This article will delve into the fascinating world of ions, exploring their formation, properties, and significance in chemistry and beyond.
Understanding Atomic Structure: The Foundation of Ion Formation
Before diving into ions, let's refresh our understanding of basic atomic structure. An atom consists of a central nucleus containing positively charged protons and neutral neutrons. Surrounding the nucleus are negatively charged electrons, arranged in energy levels or shells. The number of protons in an atom's nucleus defines its atomic number and determines its element. A neutral atom has an equal number of protons and electrons, resulting in a net charge of zero.
However, atoms are rarely content to exist in isolation. They interact with other atoms, often involving the outermost electrons, known as valence electrons. These electrons are crucial in chemical bonding, the force that holds atoms together to form molecules and compounds.
Ion Formation: The Gain and Loss of Electrons
The stability of an atom is largely determined by its electron configuration. Atoms tend to strive for a full outermost electron shell, a configuration that offers maximum stability. This drive towards stability is the driving force behind ion formation.
There are two primary ways an atom can achieve a stable electron configuration:
-
Gaining electrons: Atoms with nearly full outer shells tend to gain electrons to complete their outermost electron shell. This process results in a negative ion, also known as an anion. The extra electrons create an excess of negative charge. For example, a chlorine atom (Cl) with 7 valence electrons readily gains one electron to achieve a stable configuration of 8 valence electrons, forming a chloride anion (Cl⁻).
-
Losing electrons: Atoms with only a few valence electrons often find it easier to lose these electrons rather than gain a large number to fill their outer shell. This process results in a positive ion, also known as a cation. The loss of electrons creates an excess of positive charge. For instance, a sodium atom (Na) with one valence electron readily loses this electron to achieve a stable electron configuration, forming a sodium cation (Na⁺).
Factors Influencing Ion Formation: Electronegativity and Ionization Energy
Two key atomic properties influence an atom's tendency to gain or lose electrons:
-
Electronegativity: This measures an atom's ability to attract electrons towards itself in a chemical bond. Atoms with high electronegativity tend to gain electrons and form anions. Elements like oxygen (O) and fluorine (F) are highly electronegative.
-
Ionization Energy: This is the energy required to remove an electron from a neutral atom. Atoms with low ionization energy readily lose electrons and form cations. Alkali metals, such as sodium (Na) and potassium (K), have low ionization energies.
Ionic Bonding: The Electrostatic Attraction Between Ions
Once ions are formed, they are no longer neutral atoms. They possess a net electrical charge. The electrostatic attraction between oppositely charged ions is what constitutes ionic bonding. This strong attraction holds the ions together, forming ionic compounds. For example, sodium chloride (NaCl), common table salt, is an ionic compound formed through the electrostatic attraction between Na⁺ cations and Cl⁻ anions. The resulting ionic compound is electrically neutral, as the total positive charge from the cations balances the total negative charge from the anions.
Properties of Ionic Compounds: A Consequence of Ionic Bonding
The strong electrostatic forces in ionic compounds lead to several characteristic properties:
-
High melting and boiling points: The strong attractions between ions require significant energy to overcome, resulting in high melting and boiling points.
-
Crystalline structure: Ionic compounds typically form a regular, crystalline structure, where ions are arranged in a three-dimensional lattice. This structure maximizes the electrostatic attractions and minimizes repulsions.
-
Solubility in water: Many ionic compounds are soluble in water. Water molecules, being polar, can effectively surround and separate the ions, breaking the ionic bonds.
-
Conductivity when molten or dissolved: When molten or dissolved in water, ionic compounds conduct electricity. The mobile ions can carry electrical charge. In their solid crystalline state, the ions are fixed in the lattice, preventing electrical conductivity.
-
Brittleness: Ionic crystals are often brittle. When struck, the layers of ions can shift, leading to repulsion between like charges and causing the crystal to fracture.
Examples of Ions and Ionic Compounds: A Diverse World
Ions are ubiquitous in the natural world and essential to numerous biological and chemical processes. Here are some prominent examples:
-
Sodium ion (Na⁺): Crucial for nerve impulse transmission and fluid balance in living organisms.
-
Chloride ion (Cl⁻): A major component of bodily fluids and plays a role in maintaining osmotic balance.
-
Calcium ion (Ca²⁺): Essential for bone formation, muscle contraction, and blood clotting.
-
Potassium ion (K⁺): Involved in nerve impulse transmission and maintaining cell membrane potential.
-
Magnesium ion (Mg²⁺): Important for enzyme activity and many metabolic processes.
These are just a few examples; many other ions play vital roles in biological systems and chemical reactions.
Beyond Simple Ions: Polyatomic Ions and Complex Structures
While the examples above focus on simple monoatomic ions (ions formed from a single atom), many ions are polyatomic, meaning they consist of multiple atoms covalently bonded together carrying a net charge. Examples include:
-
Sulfate ion (SO₄²⁻): Found in many minerals and plays a role in various chemical reactions.
-
Nitrate ion (NO₃⁻): Important in fertilizers and a key component in many biological processes.
-
Phosphate ion (PO₄³⁻): Crucial for energy storage and transfer in living organisms and a component of DNA and RNA.
-
Ammonium ion (NH₄⁺): A common cation found in many fertilizers and plays a role in nitrogen cycling.
The formation and properties of these polyatomic ions are governed by the same principles of electron gain or loss and electrostatic attraction as simpler ions, but their complexity adds another layer to the study of ionic compounds.
The Significance of Ions in Various Fields
The impact of ions extends far beyond the realm of chemistry:
-
Biology: Ions are essential for life, playing crucial roles in nerve impulse transmission, muscle contraction, enzyme activity, and many other biological processes. Maintaining the correct balance of ions is critical for cellular function and overall health.
-
Medicine: Many medical treatments and diagnostic techniques rely on the properties of ions. Electrolyte imbalances can have severe consequences, and intravenous solutions often contain carefully controlled concentrations of ions to maintain fluid balance.
-
Materials Science: Ionic compounds are used extensively in materials science, finding applications in ceramics, glasses, and other materials with specific properties.
-
Environmental Science: Understanding the behavior of ions in the environment is crucial for managing water quality and addressing environmental pollution.
-
Industry: Ions are utilized in various industrial processes, including electroplating, battery technology, and the production of various chemicals.
Conclusion: The Enduring Importance of Ions
Atoms that gain or lose electrons are called ions, and these charged particles play a fundamental role in the structure and behavior of matter. From the simplest ionic compounds to the complexities of biological systems, ions exert a profound influence on the world around us. Understanding ion formation, ionic bonding, and the properties of ionic compounds is essential for anyone seeking a deeper understanding of chemistry and its applications in diverse fields. Further exploration into this fascinating area will undoubtedly uncover even more about the vital role of ions in shaping our world.
Latest Posts
Latest Posts
-
Identify The Plant Tissues In The Three Images
Mar 31, 2025
-
Thesis Statement Examples For Literary Analysis
Mar 31, 2025
-
Why Do Atoms Form Bonds With Other Atoms
Mar 31, 2025
-
What Is The Law Of Conservation Of Charge
Mar 31, 2025
-
How Many Sides Does A Parallelogram Have
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Atoms That Gain Or Lose Electrons Are Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
