How To Find Change In Internal Energy
Muz Play
Mar 26, 2025 · 7 min read
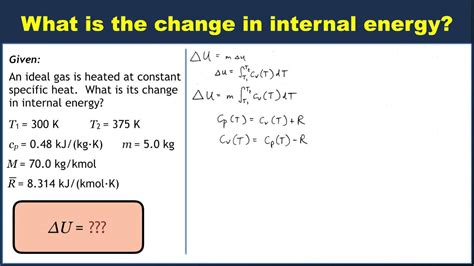
Table of Contents
How to Find Change in Internal Energy: A Comprehensive Guide
Internal energy, a fundamental concept in thermodynamics, represents the total energy stored within a system. Understanding how to determine changes in internal energy (ΔU) is crucial for analyzing various thermodynamic processes. This comprehensive guide will explore the different methods and approaches used to calculate ΔU, covering both theoretical underpinnings and practical applications.
Understanding Internal Energy and its Components
Before delving into the methods of calculating ΔU, let's solidify our understanding of internal energy itself. Internal energy (U) encompasses all forms of energy possessed by the molecules within a system. This includes:
-
Kinetic Energy: This is the energy associated with the motion of molecules, encompassing translational, rotational, and vibrational motion. The temperature of a system is directly related to the average kinetic energy of its molecules. Higher temperatures generally imply higher average kinetic energies.
-
Potential Energy: This relates to the forces of interaction between molecules. These forces can be attractive (like van der Waals forces) or repulsive, and their interplay significantly contributes to the system's overall potential energy. The potential energy depends on factors such as intermolecular distances and the nature of the molecules.
It's important to note that we cannot directly measure the absolute value of internal energy. Instead, we focus on changes in internal energy, ΔU, which is the difference between the final and initial internal energies of a system. This change is what's physically meaningful and measurable.
The First Law of Thermodynamics: The Cornerstone of ΔU Calculation
The first law of thermodynamics provides the fundamental framework for calculating changes in internal energy. It states that energy cannot be created or destroyed, only transferred or converted from one form to another. In terms of a closed system (no mass transfer), the first law can be expressed as:
ΔU = Q + W
Where:
- ΔU is the change in internal energy of the system.
- Q is the heat transferred to the system. A positive Q indicates heat added to the system, while a negative Q indicates heat removed from the system.
- W is the work done on the system. A positive W indicates work done on the system, increasing its internal energy, while a negative W indicates work done by the system, decreasing its internal energy.
This equation is incredibly powerful because it connects changes in internal energy to easily measurable quantities: heat and work. However, the specific calculation of Q and W depends heavily on the nature of the thermodynamic process involved.
Calculating ΔU for Different Thermodynamic Processes
Different thermodynamic processes involve varying relationships between heat, work, and internal energy. Let's examine some common processes:
1. Isothermal Processes (Constant Temperature)
In an isothermal process, the temperature remains constant. This implies that the average kinetic energy of the molecules stays the same. Therefore, any change in internal energy must result solely from changes in potential energy, often due to changes in volume or phase. The calculation of ΔU requires careful consideration of the work done, as it directly influences the potential energy. For an ideal gas undergoing an isothermal process, ΔU = 0 because the internal energy of an ideal gas depends only on temperature.
2. Isobaric Processes (Constant Pressure)
Isobaric processes occur at constant pressure. Here, both heat and work can contribute to the change in internal energy. The work done is easily calculated using W = -PΔV, where P is the constant pressure and ΔV is the change in volume. Knowing the heat transfer (Q) and calculating the work (W), we can directly apply the first law to find ΔU.
3. Isochoric Processes (Constant Volume)
In isochoric processes, the volume remains constant. No work is done (W = 0) because there is no change in volume (ΔV = 0). Therefore, the change in internal energy is solely due to heat transfer: ΔU = Q. This makes isochoric processes particularly simple for determining ΔU.
4. Adiabatic Processes (No Heat Transfer)
Adiabatic processes occur without any heat exchange with the surroundings (Q = 0). The change in internal energy is entirely determined by the work done: ΔU = W. In these processes, the work done causes a change in the internal energy of the system, leading to a temperature change.
5. Cyclic Processes
A cyclic process is one that returns to its initial state after a series of transformations. In a complete cycle, the change in internal energy is zero (ΔU = 0). This is a direct consequence of the state function nature of internal energy; its value depends only on the state of the system, not on the path taken. Therefore, for a complete cycle, the net heat transfer equals the negative of the net work done: Q = -W.
Beyond the First Law: Specific Heat Capacity and ΔU
For many substances, especially solids and liquids, calculating the heat transferred (Q) requires the concept of specific heat capacity (c). Specific heat capacity represents the amount of heat required to raise the temperature of 1 gram (or 1 mole) of a substance by 1 degree Celsius (or 1 Kelvin).
The equation relating heat transfer to specific heat capacity is:
Q = mcΔT
Where:
- Q is the heat transferred.
- m is the mass of the substance.
- c is the specific heat capacity of the substance.
- ΔT is the change in temperature.
By incorporating this equation into the first law, we can relate ΔU directly to temperature changes:
ΔU = mcΔT + W (for constant pressure systems)
Remember to use the appropriate specific heat capacity at constant volume (Cv) or constant pressure (Cp) depending on the process.
Practical Applications and Examples
The ability to calculate ΔU has significant applications across various fields:
-
Chemical Engineering: Determining the energy changes in chemical reactions is crucial for designing and optimizing processes. The heat of reaction is directly related to ΔU.
-
Mechanical Engineering: Analyzing the energy balance in engines and power plants requires a precise understanding of ΔU in various thermodynamic cycles.
-
Environmental Science: Studying atmospheric processes and climate change involves analyzing energy transfer and storage, directly relying on calculating changes in internal energy.
Example 1: A gas is compressed isothermally at 300 K. The work done on the gas is 100 J. What is the change in internal energy?
Since the process is isothermal, ΔU = 0. The work done on the system is 100 J, but this energy is released as heat to maintain constant temperature.
Example 2: A gas is heated at constant volume, absorbing 200 J of heat. What is the change in internal energy?
Because the volume is constant, W = 0. Therefore, ΔU = Q = 200 J. The internal energy increases by 200 J.
Example 3: 100 grams of water are heated from 20°C to 30°C at constant pressure. The specific heat capacity of water is approximately 4.18 J/g°C. Calculate the change in internal energy. Assume the expansion work is negligible in this case, so we'll primarily consider the heat transfer.
First, calculate the heat transferred: Q = mcΔT = (100 g)(4.18 J/g°C)(10°C) = 4180 J.
Since the work done is negligible, ΔU ≈ Q = 4180 J.
Advanced Considerations and Limitations
This guide provides a foundational understanding of calculating ΔU. More advanced considerations include:
-
Non-ideal gases: For non-ideal gases, the relationship between internal energy and temperature is more complex, requiring the use of more sophisticated equations of state.
-
Phase transitions: During phase transitions (e.g., melting, boiling), the internal energy changes significantly, and specific equations are needed to account for the latent heat associated with these transitions.
-
Chemical reactions: Calculating ΔU for chemical reactions necessitates considering the changes in enthalpy (ΔH) and the work involved, such as expansion work.
-
Open systems: The first law needs modification for open systems where mass transfer occurs, adding terms to account for the energy transported with the mass flow.
Conclusion
Understanding how to find change in internal energy is a cornerstone of thermodynamics, crucial for analyzing a wide variety of processes. This guide has explored the fundamental principles, presented various calculation methods for different thermodynamic processes, and highlighted practical applications. While the basic principles are relatively straightforward, remember that the complexity increases when dealing with non-ideal systems, phase transitions, and chemical reactions. A thorough understanding of the first law of thermodynamics and its applications provides the crucial foundation for mastering these advanced concepts. Remember to always carefully consider the specific conditions of the process when applying these principles.
Latest Posts
Latest Posts
-
Punnett Squares Crosses Involving One Trait
Mar 29, 2025
-
When Pressure Increases Then The Volume Must
Mar 29, 2025
-
How To Check For Linear Independence
Mar 29, 2025
-
Find The Characteristic Polynomial Of The Matrix
Mar 29, 2025
-
Atoms That Gain Or Lose Electrons Are Called
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about How To Find Change In Internal Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
