Osmosis And Diffusion Lab With Dialysis Tubing Answers
Muz Play
Mar 24, 2025 · 6 min read
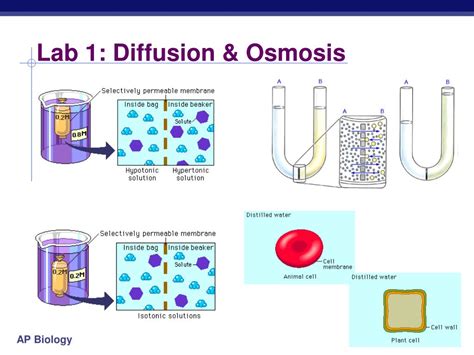
Table of Contents
Osmosis and Diffusion Lab with Dialysis Tubing: A Comprehensive Guide
Understanding osmosis and diffusion is fundamental to comprehending biological processes. This lab utilizes dialysis tubing, a semi-permeable membrane, to simulate cell membranes and explore the principles of osmosis and diffusion. This comprehensive guide will walk you through a typical osmosis and diffusion lab, provide detailed answers, and offer insights for maximizing your understanding and report writing.
Understanding Osmosis and Diffusion
Before delving into the lab procedures and results, let's solidify our understanding of the core concepts:
Diffusion
Diffusion is the net movement of particles from a region of higher concentration to a region of lower concentration. This movement continues until equilibrium is reached, meaning the concentration of particles is uniform throughout the system. The driving force behind diffusion is the inherent kinetic energy of particles; they are constantly in motion, colliding and spreading out. The rate of diffusion is influenced by factors such as temperature (higher temperature, faster diffusion), particle size (smaller particles diffuse faster), and concentration gradient (steeper gradient, faster diffusion).
Osmosis
Osmosis is a special case of diffusion involving the movement of water molecules across a selectively permeable membrane. This membrane allows the passage of water but restricts the movement of certain solutes. Water moves from a region of higher water concentration (lower solute concentration) to a region of lower water concentration (higher solute concentration) to equalize the concentration on both sides of the membrane. The driving force is the water potential, which is influenced by both solute concentration and pressure.
The Dialysis Tubing Experiment: A Simulated Cell
Dialysis tubing acts as an artificial cell membrane, allowing the passage of small molecules (like water and certain solutes) while restricting larger molecules. This makes it an ideal tool for observing osmosis and diffusion in a controlled laboratory setting.
Typical Lab Procedure: A Step-by-Step Guide
A standard osmosis and diffusion lab using dialysis tubing typically involves these steps:
-
Preparation: Obtain several lengths of dialysis tubing. Soak the tubing in water to make it pliable and increase its permeability.
-
Solution Preparation: Prepare several solutions of varying concentrations, often including distilled water (control), sucrose solution, and potentially other solutes like glucose or starch.
-
Dialysis Bag Creation: Tie one end of a piece of dialysis tubing securely. Fill each bag with a different solution, leaving some space for expansion. Securely tie the other end of the tubing.
-
Weighing: Carefully weigh each dialysis bag and record the initial mass.
-
Immersion: Immerse each dialysis bag in a beaker containing a specific solution (this solution might be the same as the inside solution or a different one, depending on your experimental design).
-
Incubation: Allow the bags to sit in their respective solutions for a predetermined amount of time (e.g., 30 minutes, 1 hour, or longer).
-
Removal and Weighing: After the incubation period, carefully remove the dialysis bags from the beakers, blot them dry, and weigh them again. Record the final mass.
-
Qualitative Tests (Optional): Depending on the solutes used, you may perform qualitative tests (like Benedict's test for glucose or iodine test for starch) to determine if these molecules moved across the dialysis tubing.
Analyzing the Results and Interpreting the Data
The change in mass of the dialysis bags provides crucial information about the movement of water. An increase in mass indicates water moved into the bag (osmosis), while a decrease indicates water moved out of the bag. The direction of water movement depends on the concentration gradient – water moves towards the solution with a higher solute concentration.
Sample Data and Interpretation
Let's consider a hypothetical experiment with three dialysis bags:
| Dialysis Bag | Initial Solution | Initial Mass (g) | Final Mass (g) | Mass Change (g) |
|---|---|---|---|---|
| Bag A | Distilled Water | 10.0 | 10.5 | +0.5 |
| Bag B | 10% Sucrose Solution | 12.0 | 11.5 | -0.5 |
| Bag C | 20% Sucrose Solution | 11.0 | 10.0 | -1.0 |
Interpretation:
-
Bag A: The bag containing distilled water gained mass. This indicates that water moved into the bag from the surrounding solution (assuming the surrounding solution was a higher solute concentration).
-
Bag B: The bag containing a 10% sucrose solution lost mass. Water moved out of the bag into the surrounding solution (assuming the surrounding solution was a lower solute concentration or pure water).
-
Bag C: The bag containing a 20% sucrose solution lost more mass than Bag B. This shows a larger water movement out of the bag due to the steeper concentration gradient.
Qualitative tests (if performed) would confirm the movement of small molecules like glucose across the membrane. The absence of starch (a large molecule) in the external solution confirms the semi-permeable nature of the dialysis tubing.
Potential Experimental Variations and Extensions
The basic experiment can be modified to explore different aspects of osmosis and diffusion:
-
Different Solutes: Using various solutes with varying molecular weights allows investigation of selective permeability.
-
Different Concentrations: Varying the concentrations inside and outside the dialysis bags allows for a detailed examination of the relationship between concentration gradient and water movement.
-
Temperature: Investigating the effect of temperature on the rate of osmosis and diffusion provides insight into the kinetic energy aspect.
-
Multiple Solutes: Including multiple solutes allows you to study the interactions and competitive movements of different molecules.
Writing Your Lab Report: Key Elements
Your lab report should be a clear and concise presentation of your experiment. Key sections include:
-
Introduction: Briefly explain osmosis and diffusion, highlighting their biological significance. State the purpose of your experiment.
-
Materials and Methods: Detail the materials used (including specific concentrations) and the step-by-step procedure followed. This section should be detailed enough for someone else to replicate your experiment.
-
Results: Present your data clearly, often using tables and graphs. Include calculations (e.g., percentage change in mass).
-
Discussion: Analyze your results. Explain your observations in relation to the principles of osmosis and diffusion. Address any unexpected results or sources of error. Compare your findings with expected outcomes based on the theory.
-
Conclusion: Summarize your findings and state whether your hypothesis was supported. Suggest further investigations or limitations of the study.
Common Sources of Error and How to Minimize Them
-
Incomplete soaking of dialysis tubing: Ensure the tubing is thoroughly soaked to allow proper permeability.
-
Leaks in the dialysis bags: Carefully tie the ends of the bags to prevent leakage.
-
Inaccurate measurements: Use accurate weighing scales and measuring equipment.
-
Incomplete drying of bags before weighing: Blot the bags gently but thoroughly to remove excess water.
Beyond the Lab: Real-World Applications
Understanding osmosis and diffusion is crucial for understanding many biological processes, including:
-
Nutrient uptake by plants: Osmosis and diffusion are essential for water and nutrient absorption from the soil.
-
Gas exchange in the lungs: Oxygen and carbon dioxide diffuse across the alveoli in the lungs.
-
Waste removal in the kidneys: Filtration and reabsorption in the kidneys rely on these principles.
-
Nutrient absorption in the digestive system: Nutrients are absorbed through the intestinal lining via diffusion and osmosis.
By carefully executing this lab experiment and thoroughly analyzing your results, you will gain a solid practical understanding of osmosis and diffusion – fundamental processes that are essential to life itself. Remember to apply critical thinking, meticulous data collection, and clear communication in your lab report to effectively demonstrate your comprehension of these vital concepts.
Latest Posts
Latest Posts
-
Elements With Similar Chemical Properties Are Found
Mar 26, 2025
-
How Do You Calculate An Index
Mar 26, 2025
-
Difference Between Meiosis I And Ii
Mar 26, 2025
-
A Van Der Waals Interaction Is The Weak Attraction Between
Mar 26, 2025
-
Which Element Is Important In Directly Triggering Contraction
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Osmosis And Diffusion Lab With Dialysis Tubing Answers . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
