Elements With Similar Chemical Properties Are Found
Muz Play
Mar 26, 2025 · 6 min read
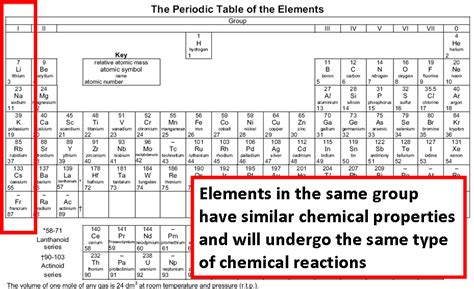
Table of Contents
Elements with Similar Chemical Properties are Found in the Same Group of the Periodic Table
The periodic table, a cornerstone of chemistry, organizes elements not haphazardly, but based on their recurring chemical and physical properties. This organization reveals a fundamental truth: elements with similar chemical properties are found in the same group (or family) of the periodic table. This isn't a coincidence; it's a direct consequence of the underlying structure of atoms and how their electrons are arranged. Understanding this principle is crucial for predicting the behavior of elements and their compounds. This article delves deep into this concept, exploring the reasons behind this similarity, the exceptions, and its implications.
The Role of Electron Configuration
The key to understanding why elements in the same group exhibit similar chemical properties lies in their electron configuration. The electron configuration describes how electrons are distributed among the various energy levels and sublevels within an atom. Specifically, it's the valence electrons, the electrons in the outermost energy level, that determine an element's chemical behavior.
Valence Electrons: The Chemical Actors
Valence electrons are the primary participants in chemical bonding. They are responsible for forming chemical bonds with other atoms, thereby determining the element's reactivity and the types of compounds it can form. Elements within the same group possess the same number of valence electrons. This shared number of valence electrons directly translates into similar chemical properties.
For instance, consider Group 1, the alkali metals (lithium, sodium, potassium, rubidium, cesium, and francium). Each of these elements has one valence electron. This single valence electron is easily lost, resulting in the formation of +1 ions. This shared characteristic leads to striking similarities in their reactivity: they all readily react with water, producing hydrogen gas and a metal hydroxide. Their similar reactivity is directly attributable to their identical number of valence electrons.
Similarly, Group 17, the halogens (fluorine, chlorine, bromine, iodine, and astatine), all have seven valence electrons. They tend to gain one electron to achieve a stable octet (eight electrons in their outermost shell), forming -1 ions. This shared tendency leads to similar chemical properties, such as their high reactivity and their ability to form salts with alkali metals.
Periodic Trends and Group Properties
The periodic table isn't merely a list; it showcases periodic trends – gradual changes in properties as you move across a period or down a group. These trends are directly linked to the changes in electron configuration and nuclear charge.
Down a Group: Increasing Atomic Radius and Decreasing Ionization Energy
As you move down a group, the atomic radius increases. This is because additional electron shells are added, increasing the distance between the valence electrons and the nucleus. Consequently, the effective nuclear charge (the net positive charge experienced by the valence electrons) decreases. This weaker attraction between the nucleus and valence electrons leads to a decrease in ionization energy – the energy required to remove an electron from an atom. This lower ionization energy explains why elements lower in a group are generally more reactive than those higher up.
Across a Period: Decreasing Atomic Radius and Increasing Ionization Energy
Across a period, the atomic radius generally decreases. While additional electrons are added, they are added to the same energy level. The increasing nuclear charge pulls the electrons closer, resulting in a smaller atomic radius. Simultaneously, the ionization energy generally increases because the stronger nuclear charge holds the valence electrons more tightly. This increasing ionization energy correlates with a decrease in reactivity.
Exceptions to the Rule: The Subtleties of Chemical Behavior
While the general rule holds true, there are exceptions and nuances to consider. The simple valence electron count doesn't tell the whole story. Other factors influence chemical properties, including:
-
Shielding effect: Inner electrons shield the valence electrons from the full positive charge of the nucleus. This shielding effect modifies the effective nuclear charge experienced by the valence electrons. Elements with different electron configurations in the inner shells may experience variations in shielding, leading to subtle differences in chemical behavior even within the same group.
-
Electron-electron repulsion: As the number of electrons increases, the repulsion between them becomes more significant. This repulsion can slightly alter the energy levels and affect the reactivity of the element.
-
Relativistic effects: At very high atomic numbers, relativistic effects become significant. These effects, arising from the high speeds of inner electrons, can alter the size and energy levels of orbitals, leading to unexpected deviations in chemical properties. This is particularly noticeable in the heavier elements.
-
d-block and f-block elements: The transition metals (d-block elements) and inner transition metals (f-block elements) display more complex behavior due to the involvement of d and f electrons in bonding. While they exhibit some group trends, the variety of oxidation states and the participation of d and f electrons lead to greater diversity in their chemical properties compared to the main group elements (s-block and p-block).
Implications and Applications
The principle that elements with similar chemical properties are found in the same group has profound implications across various fields:
-
Predicting chemical behavior: The periodic table allows chemists to predict the likely reactivity and bonding behavior of elements based on their position. This predictive power is crucial in designing new materials and chemical reactions.
-
Material science: Understanding group properties is essential for designing materials with specific characteristics. For instance, the alkali metals' reactivity is exploited in various applications, while the inertness of noble gases (Group 18) makes them valuable in applications requiring a non-reactive atmosphere.
-
Geochemistry: The distribution and behavior of elements in the Earth's crust and other geological environments are largely dictated by their chemical properties, which are directly related to their group placement.
-
Biochemistry: Many biological processes rely on the specific chemical properties of elements. For example, the role of alkali metals (like sodium and potassium) in nerve impulse transmission and the role of transition metals (like iron and copper) in enzymatic reactions highlight the importance of group properties in biological systems.
Conclusion: A Fundamental Principle in Chemistry
The organization of the periodic table, based on recurring chemical properties, is a testament to the underlying order in the structure of matter. The principle that elements with similar chemical properties are found in the same group is a fundamental concept in chemistry. While exceptions and nuances exist, understanding the relationship between electron configuration, valence electrons, and chemical behavior remains crucial for predicting and manipulating the behavior of elements and their compounds. This knowledge underpins countless scientific and technological advancements, underscoring the enduring importance of the periodic table as a tool for understanding the chemical world. Further exploration into the intricacies of electron interactions and the factors that subtly modify periodic trends will continue to refine our understanding of this fundamental principle.
Latest Posts
Latest Posts
-
Find The Characteristic Polynomial Of The Matrix
Mar 29, 2025
-
Atoms That Gain Or Lose Electrons Are Called
Mar 29, 2025
-
Why Is The Electric Field Inside A Conductor Zero
Mar 29, 2025
-
Solving Log And Exponential Equations Worksheet
Mar 29, 2025
-
What I Can Learn From Atomic Structures
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Elements With Similar Chemical Properties Are Found . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
