What I Can Learn From Atomic Structures
Muz Play
Mar 29, 2025 · 6 min read
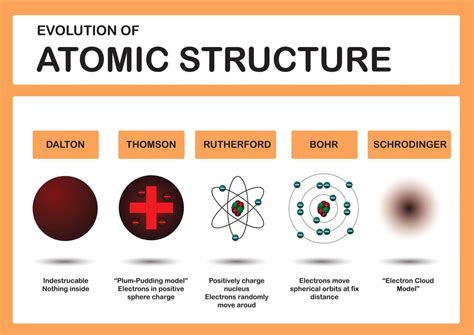
Table of Contents
What I Can Learn From Atomic Structures: A Journey into the Fundamentals of Matter
The seemingly simple concept of atomic structure, the building blocks of all matter, holds a wealth of knowledge that extends far beyond the realm of chemistry and physics. Understanding atomic structure isn't just about memorizing electron configurations; it's about grasping fundamental principles that permeate various aspects of our lives, from the technology we use daily to the very fabric of the universe. This article delves deep into the fascinating world of atomic structure, exploring its implications and lessons applicable to diverse fields and personal growth.
Understanding the Building Blocks: A Primer on Atomic Structure
Before we explore the broader implications, let's briefly recap the core components of an atom:
- Protons: Positively charged particles residing in the atom's nucleus. The number of protons defines the element.
- Neutrons: Neutral particles also found in the nucleus. Their number can vary within the same element, resulting in isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. The arrangement of electrons determines the atom's chemical properties and reactivity.
The nucleus, containing protons and neutrons, makes up the vast majority of the atom's mass, while the electrons occupy the vast majority of its volume. This seemingly simple model, however, reveals complexities and underlying principles that offer profound lessons.
Lesson 1: The Power of Fundamental Building Blocks
The atom's structure beautifully illustrates the principle of emergent properties. Individual protons, neutrons, and electrons are simple entities, yet their specific arrangement and interactions give rise to the incredible diversity of matter we observe in the universe. From the inert gases to the reactive metals, the differences stem from subtle variations in the number and arrangement of these subatomic particles. This teaches us that complex systems often emerge from simple components, a principle applicable to many aspects of life, from the intricate workings of the human body to the evolution of complex societies.
Applying this lesson:
- Project Management: Understanding that complex projects are built from smaller, manageable tasks.
- Teamwork: Recognizing individual contributions' crucial role in the success of a larger team goal.
- Problem Solving: Breaking down complex problems into smaller, more manageable parts.
Lesson 2: Stability and Equilibrium: The Importance of Balance
Atomic stability is directly related to the arrangement of electrons. Atoms strive for a stable electron configuration, often achieving this by gaining, losing, or sharing electrons with other atoms. This drive for stability manifests in chemical bonding, the force that holds atoms together to form molecules. This teaches us the importance of balance and equilibrium in achieving stability and achieving goals. An imbalance, whether in the atom's electron configuration or in our lives, can lead to instability and reactivity.
Applying this lesson:
- Personal Well-being: Maintaining a balance between work, rest, and social life.
- Financial Planning: Achieving financial stability through careful budgeting and saving.
- Relationship Dynamics: Maintaining a healthy balance of power and reciprocity in relationships.
Lesson 3: Adaptability and Change: Isotopes and Radioactive Decay
Isotopes, atoms of the same element with differing numbers of neutrons, demonstrate the concept of variability and adaptability. While sharing the same fundamental properties, isotopes can exhibit different physical and chemical behaviors. Radioactive isotopes, in particular, undergo radioactive decay, transforming into different elements over time. This highlights the dynamic nature of matter and the constant state of flux within the universe. The lesson here is that change is inevitable and adapting to it is crucial for survival and growth.
Applying this lesson:
- Career Development: Adapting to changing job markets and acquiring new skills.
- Personal Growth: Embracing challenges and learning from failures.
- Technological Advancement: Recognizing that technology constantly evolves and requires adaptation.
Lesson 4: The Quantum Realm: Uncertainty and Probability
The behavior of electrons is governed by the principles of quantum mechanics, introducing the concepts of uncertainty and probability. We can't precisely determine both the position and momentum of an electron simultaneously. Instead, we deal with probabilities and wave functions, describing the likelihood of finding an electron in a particular region of space. This challenges our classical intuition and teaches us to embrace the inherent uncertainty in many aspects of life.
Applying this lesson:
- Decision-Making: Accepting that decisions often involve risks and uncertainty.
- Goal Setting: Understanding that unexpected events can impact our progress.
- Investing: Acknowledging that market fluctuations and risk are inherent in investment strategies.
Lesson 5: The Power of Interactions: Chemical Bonding and Molecular Structures
Atoms don't exist in isolation. They interact with each other, forming chemical bonds that lead to the formation of molecules and complex structures. The type of bond (ionic, covalent, metallic) dictates the properties of the resulting compound, influencing its physical and chemical characteristics. This underscores the importance of collaboration and interaction in achieving greater complexity and functionality.
Applying this lesson:
- Collaboration: Recognizing the power of teamwork and synergy in achieving common goals.
- Networking: Building relationships to expand opportunities and gain new perspectives.
- Business Partnerships: Understanding the importance of synergistic relationships for mutual success.
Lesson 6: From Atoms to the Cosmos: The Scale and Scope of the Universe
The study of atomic structure provides a window into the vastness of the universe. The same fundamental forces and principles that govern the interactions of atoms also operate on a cosmic scale, shaping stars, galaxies, and planetary systems. This emphasizes the interconnectedness of all things, highlighting the intricate web of relationships that govern the universe.
Applying this lesson:
- Environmental Awareness: Recognizing our impact on the environment and the interconnectedness of ecosystems.
- Global Citizenship: Understanding our role in the global community and the importance of international cooperation.
- Spiritual Understanding: Appreciating the interconnectedness of all living things and our place within the universe.
Lesson 7: The Pursuit of Knowledge: The Scientific Method in Action
The understanding of atomic structure is a testament to the power of the scientific method. Through meticulous experimentation, observation, and theoretical development, scientists have gradually unravelled the mysteries of the atom. This underscores the importance of continuous learning, critical thinking, and evidence-based reasoning.
Applying this lesson:
- Lifelong Learning: Embracing continuous learning and personal development.
- Critical Thinking: Developing skills to analyze information objectively and make informed decisions.
- Problem Solving: Applying the scientific method to tackle challenges effectively.
Conclusion: A Universe of Lessons from the Subatomic Realm
The study of atomic structure offers far more than just a deeper understanding of chemistry and physics. It provides profound lessons about the nature of matter, the importance of balance and change, the power of interactions, and the vast scope of the universe. By applying these principles to various aspects of our lives, we can enhance our understanding of the world around us and unlock our potential for personal and collective growth. The atom, in its simplicity and complexity, serves as a powerful microcosm of the universe and a constant source of inspiration for learning and discovery. The more we learn about atomic structure, the more we learn about ourselves and our place in the grand tapestry of existence. This continuous exploration not only benefits scientific advancement but enriches our understanding of the universe and inspires innovation in various fields. The seemingly small world of the atom holds immense potential for discovery, and its lessons continue to shape our world in profound ways.
Latest Posts
Latest Posts
-
How Many Sides Does A Parallelogram Have
Mar 31, 2025
-
Elements That Are Gases At Room Temperature
Mar 31, 2025
-
How Did Abraham Lincolns Assassination Affect Reconstruction
Mar 31, 2025
-
How To Construct A Standard Curve On Excel
Mar 31, 2025
-
Does A Gas Have A Fixed Volume
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What I Can Learn From Atomic Structures . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
